Antimicrobial peptides effectively kill a broad spectrum of Listeria monocytogenes and Staphylococcus aureus strains independently of origin, sub-type, or virulence factor expression
- PMID: 19036162
- PMCID: PMC2639596
- DOI: 10.1186/1471-2180-8-205
Antimicrobial peptides effectively kill a broad spectrum of Listeria monocytogenes and Staphylococcus aureus strains independently of origin, sub-type, or virulence factor expression
Abstract
Background: Host defense peptides (HDPs), or antimicrobial peptides (AMPs), are important components of the innate immune system that bacterial pathogens must overcome to establish an infection and HDPs have been suggested as novel antimicrobial therapeutics in treatment of infectious diseases. Hence it is important to determine the natural variation in susceptibility to HDPs to ensure a successful use in clinical treatment regimes.
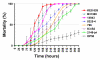
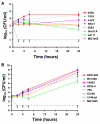
Results: Strains of two human bacterial pathogens, Listeria monocytogenes and Staphylococcus aureus, were selected to cover a wide range of origin, sub-type, and phenotypic behavior. Strains within each species were equally sensitive to HDPs and oxidative stress representing important components of the innate immune defense system. Four non-human peptides (protamine, plectasin, novicidin, and novispirin G10) were similar in activity profile (MIC value spectrum) to the human beta-defensin 3 (HBD-3). All strains were inhibited by concentrations of hydrogen peroxide between 0.1% - 1.0%. Sub-selections of both species differed in expression of several virulence-related factors and in their ability to survive in human whole blood and kill the nematode virulence model Caenorhabditis elegans. For L. monocytogenes, proliferation in whole blood was paralleled by high invasion in Caco-2 cells and fast killing of C. elegans, however, no such pattern in phenotypic behavior was observed for S. aureus and none of the phenotypic differences were correlated to sensitivity to HDPs.
Conclusion: Strains of L. monocytogenes and S. aureus were within each species equally sensitive to a range of HDPs despite variations in subtype, origin, and phenotypic behavior. Our results suggest that therapeutic use of HDPs will not be hampered by occurrence of naturally tolerant strains of the two species investigated in the present study.
Figures
Similar articles
-
The heme sensing response regulator HssR in Staphylococcus aureus but not the homologous RR23 in Listeria monocytogenes modulates susceptibility to the antimicrobial peptide plectasin.BMC Microbiol. 2010 Dec 1;10:307. doi: 10.1186/1471-2180-10-307. BMC Microbiol. 2010. PMID: 21122114 Free PMC article.
-
Susceptibility of Listeria monocytogenes to antimicrobial peptides.FEMS Microbiol Lett. 2003 Sep 12;226(1):101-5. doi: 10.1016/S0378-1097(03)00579-2. FEMS Microbiol Lett. 2003. PMID: 13129614
-
The GraS Sensor in Staphylococcus aureus Mediates Resistance to Host Defense Peptides Differing in Mechanisms of Action.Infect Immun. 2015 Nov 23;84(2):459-66. doi: 10.1128/IAI.01030-15. Print 2016 Feb. Infect Immun. 2015. PMID: 26597988 Free PMC article.
-
Antimicrobial and host defense peptides for therapeutic use against multidrug-resistant pathogens: new hope on the horizon.IDrugs. 2009 Jun;12(6):376-80. IDrugs. 2009. PMID: 19517318 Review.
-
Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases.Exp Dermatol. 2017 Nov;26(11):989-998. doi: 10.1111/exd.13314. Epub 2017 Apr 27. Exp Dermatol. 2017. PMID: 28191680 Review.
Cited by
-
Human beta-defensin-3 producing cells in septic implant loosening.J Mater Sci Mater Med. 2015 Feb;26(2):98. doi: 10.1007/s10856-015-5440-4. Epub 2015 Feb 6. J Mater Sci Mater Med. 2015. PMID: 25655501
-
Synergistic interactions between antimicrobial peptides derived from plectasin and lipid nanocapsules containing monolaurin as a cosurfactant against Staphylococcus aureus.Int J Nanomedicine. 2017 Aug 8;12:5687-5699. doi: 10.2147/IJN.S139625. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28848347 Free PMC article.
-
Reverse micelle-lipid nanocapsules: a novel strategy for drug delivery of the plectasin derivate AP138 antimicrobial peptide.Int J Nanomedicine. 2018 Nov 15;13:7565-7574. doi: 10.2147/IJN.S180040. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30532539 Free PMC article.
-
Resistance and tolerance to tropodithietic acid, an antimicrobial in aquaculture, is hard to select.Antimicrob Agents Chemother. 2011 Apr;55(4):1332-7. doi: 10.1128/AAC.01222-10. Epub 2011 Jan 24. Antimicrob Agents Chemother. 2011. PMID: 21263047 Free PMC article.
-
Targeting listeria monocytogenes rpoA and rpoD genes using peptide nucleic acids.Nucleic Acid Ther. 2013 Oct;23(5):363-7. doi: 10.1089/nat.2013.0426. Epub 2013 Jul 16. Nucleic Acid Ther. 2013. PMID: 23859300 Free PMC article.
References
-
- Marshall SH, Arenas G. Antimicrobial peptides: A natural alternative to chemical antibiotics and a potential for applied biotechnology. Elec J Biotechnol. 2003;6:271–284.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

