Platelets actively sequester angiogenesis regulators
- PMID: 19036702
- PMCID: PMC2661866
- DOI: 10.1182/blood-2008-06-159541
Platelets actively sequester angiogenesis regulators
Abstract
Clinical trials with antiangiogenic agents have not been able to validate plasma or serum levels of angiogenesis regulators as reliable markers of cancer presence or therapeutic response. We recently reported that platelets contain numerous proteins that regulate angiogenesis. We now show that accumulation of angiogenesis regulators in platelets of animals bearing malignant tumors exceeds significantly their concentration in plasma or serum, as well as their levels in platelets from non-tumor-bearing animals. This process is selective, as platelets do not take up a proportional amount of other plasma proteins (eg, albumin), even though these may be present at higher concentrations. We also find that VEGF-enriched Matrigel pellets implanted subcutaneously into mice or the minute quantities of VEGF secreted by microscopic subcutaneous tumors (0.5-1 mm(3)) result in an elevation of VEGF levels in platelets, without any changes in its plasma levels. The profile of other angiogenesis regulatory proteins (eg, platelet-derived growth factor, basic fibroblast growth factor) sequestered by platelets also reflects the presence of tumors in vivo before they can be macroscopically evident. The ability of platelets to selectively take up angiogenesis regulators in cancer-bearing hosts may have implications for the diagnosis and management of many angiogenesis-related diseases and provide a guide for antiangiogenic therapies.
Figures
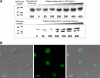
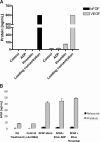

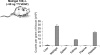

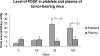
Comment in
-
To give and take - life of a platelet.Blood. 2009 Mar 19;113(12):2617. doi: 10.1182/blood-2009-01-198135. Blood. 2009. PMID: 19299653 No abstract available.
References
-
- Kim HK, Song KS, Park YS, et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39:184–191. - PubMed
-
- Karpatkin S. Tumor growth and metastasis. In: Michelson AD, editor. Platelets. San Diego, CA: Academic Press; 2002. pp. 491–502.
-
- Verheul HM, Pinedo HM. Tumor growth: a putative role for platelets? Oncologist. 1998;3:II. - PubMed
-
- Folkman J, Browder T, Palmblad J. Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost. 2001;86:23–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

