Missense mutations that cause Van der Woude syndrome and popliteal pterygium syndrome affect the DNA-binding and transcriptional activation functions of IRF6
- PMID: 19036739
- PMCID: PMC2638798
- DOI: 10.1093/hmg/ddn381
Missense mutations that cause Van der Woude syndrome and popliteal pterygium syndrome affect the DNA-binding and transcriptional activation functions of IRF6
Erratum in
- Hum Mol Genet.2009 Apr 15;18(8):1544
Abstract
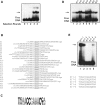
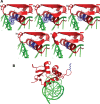
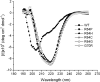
Cleft lip and cleft palate (CLP) are common disorders that occur either as part of a syndrome, where structures other than the lip and palate are affected, or in the absence of other anomalies. Van der Woude syndrome (VWS) and popliteal pterygium syndrome (PPS) are autosomal dominant disorders characterized by combinations of cleft lip, CLP, lip pits, skin-folds, syndactyly and oral adhesions which arise as the result of mutations in interferon regulatory factor 6 (IRF6). IRF6 belongs to a family of transcription factors that share a highly conserved N-terminal, DNA-binding domain and a less well-conserved protein-binding domain. To date, mutation analyses have suggested a broad genotype-phenotype correlation in which missense and nonsense mutations occurring throughout IRF6 may cause VWS; in contrast, PPS-causing mutations are highly associated with the DNA-binding domain, and appear to preferentially affect residues that are predicted to interact directly with the DNA. Nevertheless, this genotype-phenotype correlation is based on the analysis of structural models rather than on the investigation of the DNA-binding properties of IRF6. Moreover, the effects of mutations in the protein interaction domain have not been analysed. In the current investigation, we have determined the sequence to which IRF6 binds and used this sequence to analyse the effect of VWS- and PPS-associated mutations in the DNA-binding domain of IRF6. In addition, we have demonstrated that IRF6 functions as a co-operative transcriptional activator and that mutations in the protein interaction domain of IRF6 disrupt this activity.
Figures
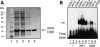

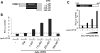
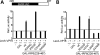
References
-
- Vanderas A.P. Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. Cleft Palate J. 1987;24:216–225. - PubMed
-
- Murray J.C., Daack-Hirsch S., Buetow K.H., Munger R., Espina L., Paglinawan N., Villanueva E., Rary J., Magee K., Magee W. Clinical and epidemiologic studies of cleft lip and palate in the Philippines. Cleft Palate Craniofac. J. 1997;34:7–10. - PubMed
-
- Neilson D.E., Brunger J.W., Heeger S., Bamshad M., Robin N.H. Mixed clefting type in Rapp-Hodgkin syndrome. Am. J. Med. Genet. 2002;108:281–284. - PubMed
-
- Jones M.C. Etiology of facial clefts: prospective evaluation of 428 patients. Cleft Palate J. 1988;25:16–20. - PubMed
-
- Suzuki K., Hu D., Bustos T., Zlotogora J., Richieri-Costa A., Helms J.A., Spritz R.A. Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat. Genet. 2000;25:427–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

