Function and ribosomal localization of aIF6, a translational regulator shared by archaea and eukarya
- PMID: 19036786
- PMCID: PMC2615626
- DOI: 10.1093/nar/gkn959
Function and ribosomal localization of aIF6, a translational regulator shared by archaea and eukarya
Abstract
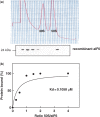
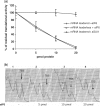
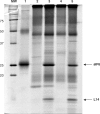
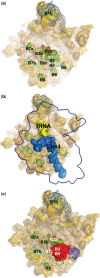
The translation factor IF6 is shared by the Archaea and the Eukarya, but is not found in Bacteria. The properties of eukaryal IF6 (eIF6) have been extensively studied, but remain somewhat elusive. eIF6 behaves as a ribosome-anti-association factor and is involved in miRNA-mediated gene silencing; however, it also seems to participate in ribosome synthesis and export. Here we have determined the function and ribosomal localization of the archaeal (Sulfolobus solfataricus) IF6 homologue (aIF6). We find that aIF6 binds specifically to the 50S ribosomal subunits, hindering the formation of 70S ribosomes and strongly inhibiting translation. aIF6 is uniformly expressed along the cell cycle, but it is upregulated following both cold- and heat shock. The aIF6 ribosomal binding site lies in the middle of the 30-S interacting surface of the 50S subunit, including a number of critical RNA and protein determinants involved in subunit association. The data suggest that the IF6 protein evolved in the archaeal-eukaryal lineage to modulate translational efficiency under unfavourable environmental conditions, perhaps acquiring additional functions during eukaryotic evolution.
Figures

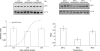

References
-
- Russell DW, Spremulli LL. Purification and characterization of a ribosome dissociation factor (eukaryotic initiation factor 6) from wheat germ. J. Biol. Chem. 1979;254:8796–8800. - PubMed
-
- Valenzuela DM, Chaudhuri A, Maitra U. Eukaryotic ribosomal subunit anti-association activity of calf liver is contained in a single polypeptide chain protein of Mr = 25,500 (eukaryotic initiation factor 6) J. Biol. Chem. 1982;257:7712–7719. - PubMed
-
- Basu U, Si K, Deng H, Maitra U. Phosphorylation of mammalian eukaryotic translation initiation factor 6 and its Saccharomyces cerevisiae homologue Tif6p: evidence that phosphorylation of Tif6p regulates its nucleocytoplasmic distribution and is required for yeast cell growth. Mol. Cell Biol. 2003;23:6187–6199. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

