A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm
- PMID: 19036802
- PMCID: PMC2685963
- DOI: 10.1242/dev.026583
A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm
Abstract
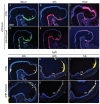
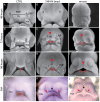
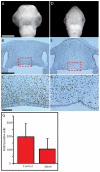
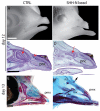
Interactions among the forebrain, neural crest and facial ectoderm regulate development of the upper jaw. To examine these interactions, we activated the Sonic hedgehog (SHH) pathway in the brain. Beginning 72 hours after activation of the SHH pathway, growth within the avian frontonasal process (FNP) was exaggerated in lateral regions and impaired in medial regions. This growth pattern is similar to that in mice and superimposed a mammalian-like morphology on the upper jaw. Jaw growth is controlled by signals from the frontonasal ectodermal zone (FEZ), and the divergent morphologies that characterize birds and mammals are accompanied by changes in the FEZ. In chicks there is a single FEZ spanning the FNP, but in mice both median nasal processes have a FEZ. In treated chicks, the FEZ was split into right and left domains that resembled the pattern present in mice. Additionally, we observed that, in the brain, fibroblast growth factor 8 (Fgf8) was downregulated, and signals in or near the nasal pit were altered. Raldh2 expression was expanded, whereas Fgf8, Wnt4, Wnt6 and Zfhx1b were downregulated. However, Wnt9b, and activation of the canonical WNT pathway, were unaltered in treated embryos. At later time points the upper beak was shortened owing to hypoplasia of the skeleton, and this phenotype was reproduced when we blocked the FGF pathway. Thus, the brain establishes multiple signaling centers within the developing upper jaw. Changes in organization of the brain that occur during evolution or as a result of disease can alter these centers and thereby generate morphological variation.
Figures



References
-
- Abzhanov, A. and Tabin, C. J. (2004). Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev. Biol. 273, 134-148. - PubMed
-
- Abzhanov, A., Protas, M., Grant, B. R., Grant, P. R. and Tabin, C. J. (2004). Bmp4 and morphological variation of beaks in Darwin's finches. Science 305, 1462-1465. - PubMed
-
- Albrecht, U. E. G., Helms, J. A. and Lin, H. (1997). Visualization of gene expression patterns by in situ hybridization. In Molecular and Cellular Methods in Developmental Toxicology (ed. G. P. Daston), pp. 23-48. Boca Raton, FL: CRC Press.
-
- Ashique, A. M., Fu, K. and Richman, J. M. (2002). Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development 129, 4647-4660. - PubMed
-
- Benouaiche, L., Gitton, Y., Vincent, C., Couly, G. and Levi, G. (2008). Sonic hedgehog signalling from foregut endoderm patterns the avian nasal capsule. Development 135, 2221-2225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

