Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors
- PMID: 19036984
- PMCID: PMC2605967
- DOI: 10.1523/JNEUROSCI.4629-08.2008
Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors
Abstract
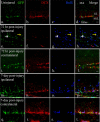
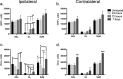
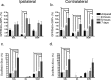
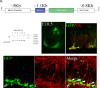
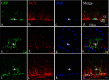
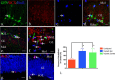
It is becoming increasingly clear that brain injuries from a variety of causes stimulate neurogenesis within the hippocampus. It remains unclear, however, how robust this response may be and what primary cell types are involved. Here, using a controlled cortical impact model of traumatic brain injury on a previously characterized transgenic mouse line that expresses enhanced green fluorescent protein (eGFP) under the control of the nestin promoter, we demonstrate that it is the earliest type-1 quiescent progenitor cells that are induced to proliferate and migrate outside the subgranular layer of the dentate gyrus. This type-1 cell activation occurs at the same time that we observe adjacent but more differentiated doublecortin-expressing progenitors (type-2 cells) being eliminated. Also, although type-2 cells remain intact in the contralateral (uninjured) dentate gyrus, the type-1 cells there are also activated and result in increased numbers of the doublecortin-expressing type-2 cells. In addition, we have generated a novel mouse transgenic that expresses a modified version of the herpes simplex virus thymidine kinase along with eGFP that allows for the visualization and inducible ablation of early dividing progenitors by exposing them to ganciclovir. Using this transgenic in the context of traumatic brain injury, we demonstrate that these early progenitors are required for injury-induced remodeling to occur. This work suggests that injury-induced hippocampal remodeling following brain injury likely requires sustained activation of quiescent early progenitors.
Figures


References
-
- Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. - PubMed
-
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. - PubMed
-
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
