Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions
- PMID: 19036987
- PMCID: PMC2628555
- DOI: 10.1523/JNEUROSCI.3038-08.2008
Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions
Abstract
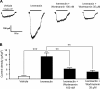
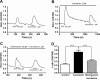
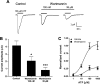
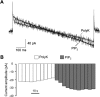
P2X receptors are ATP-gated nonselective cation channels highly permeable to calcium that contribute to nociception and inflammatory responses. The P2X(4) subtype, upregulated in activated microglia, is thought to play a critical role in the development of tactile allodynia following peripheral nerve injury. Posttranslational regulation of P2X(4) function is crucial to the cellular mechanisms of neuropathic pain, however it remains poorly understood. Here, we show that the phosphoinositides PI(4,5)P(2) (PIP(2)) and PI(3,4,5)P(3) (PIP(3)), products of phosphorylation by wortmannin-sensitive phosphatidylinositol 4-kinases and phosphatidylinositol 3-kinases, can modulate the function of native and recombinant P2X(4) receptor channels. In BV-2 microglial cells, depleting the intracellular levels of PIP(2) and PIP(3) with wortmannin significantly decreased P2X(4) current amplitude and P2X(4)-mediated calcium entry measured in patch clamp recordings and ratiometric ion imaging, respectively. Wortmannin-induced depletion of phosphoinositides in Xenopus oocytes decreased the current amplitude of P2X(4) responses by converting ATP into a partial agonist. It also decreased their recovery from desensitization and affected their kinetics. Injection of phosphoinositides in wortmannin-treated oocytes reversed these effects and application of PIP(2) on excised inside-out macropatches rescued P2X(4) currents from rundown. Moreover, we report the direct interaction of phospholipids with the proximal C-terminal domain of P2X(4) subunit (Cys(360)-Val(375)) using an in vitro binding assay. These results demonstrate novel regulatory roles of the major signaling phosphoinositides PIP(2) and PIP(3) on P2X(4) function through direct channel-lipid interactions.
Figures



References
-
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. - PubMed
-
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. - PubMed
-
- Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem. 2006;281:15044–15049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
