Fibrillin assembly requires fibronectin
- PMID: 19037100
- PMCID: PMC2633374
- DOI: 10.1091/mbc.e08-08-0830
Fibrillin assembly requires fibronectin
Abstract
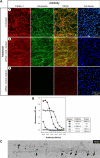
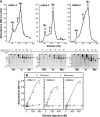
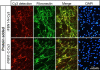
Fibrillins constitute the major backbone of multifunctional microfibrils in elastic and nonelastic extracellular matrices. Proper assembly mechanisms are central to the formation and function of these microfibrils, and their properties are often compromised in pathological circumstances such as in Marfan syndrome and in other fibrillinopathies. Here, we have used human dermal fibroblasts to analyze the assembly of fibrillin-1 in dependence of other matrix-forming proteins. siRNA knockdown experiments demonstrated that the assembly of fibrillin-1 is strictly dependent on the presence of extracellular fibronectin fibrils. Immunolabeling performed at the light and electron microscopic level showed colocalization of fibrillin-1 with fibronectin fibrils at the early stages of the assembly process. Protein-binding assays demonstrated interactions of fibronectin with a C-terminal region of fibrillin-1, -2, and -3 and with an N-terminal region of fibrillin-1. The C-terminal half of fibrillin-2 and -3 had propensities to multimerize, as has been previously shown for fibrillin-1. The C-terminal of all three fibrillins interacted strongly with fibronectin as multimers, but not as monomers. Mapping studies revealed that the major binding interaction between fibrillins and fibronectin involves the collagen/gelatin-binding region between domains FNI(6) and FNI(9).
Figures







Similar articles
-
Complex contributions of fibronectin to initiation and maturation of microfibrils.Biochem J. 2013 Dec 1;456(2):283-95. doi: 10.1042/BJ20130699. Biochem J. 2013. PMID: 24070235 Free PMC article.
-
Biogenesis of extracellular microfibrils: Multimerization of the fibrillin-1 C terminus into bead-like structures enables self-assembly.Proc Natl Acad Sci U S A. 2008 May 6;105(18):6548-53. doi: 10.1073/pnas.0706335105. Epub 2008 Apr 30. Proc Natl Acad Sci U S A. 2008. PMID: 18448684 Free PMC article.
-
Heparin/heparan sulfate controls fibrillin-1, -2 and -3 self-interactions in microfibril assembly.FEBS Lett. 2014 Aug 25;588(17):2890-7. doi: 10.1016/j.febslet.2014.06.061. Epub 2014 Jul 14. FEBS Lett. 2014. PMID: 25034023
-
Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins.Adv Exp Med Biol. 2014;802:31-47. doi: 10.1007/978-94-007-7893-1_3. Adv Exp Med Biol. 2014. PMID: 24443019 Review.
-
Microfibrils: a cornerstone of extracellular matrix and a key to understand Marfan syndrome.Ital J Anat Embryol. 2009 Oct-Dec;114(4):201-24. Ital J Anat Embryol. 2009. PMID: 20578676 Review.
Cited by
-
Collagen as a Biomaterial for Skin and Corneal Wound Healing.J Funct Biomater. 2022 Nov 16;13(4):249. doi: 10.3390/jfb13040249. J Funct Biomater. 2022. PMID: 36412890 Free PMC article. Review.
-
Proteolysis of fibrillin-2 microfibrils is essential for normal skeletal development.Elife. 2022 May 3;11:e71142. doi: 10.7554/eLife.71142. Elife. 2022. PMID: 35503090 Free PMC article.
-
Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase.Proc Natl Acad Sci U S A. 2009 Nov 10;106(45):19029-34. doi: 10.1073/pnas.0908268106. Epub 2009 Oct 23. Proc Natl Acad Sci U S A. 2009. PMID: 19855011 Free PMC article.
-
Live Imaging of Type I Collagen Assembly Dynamics in Osteoblasts Stably Expressing GFP and mCherry-Tagged Collagen Constructs.J Bone Miner Res. 2018 Jun;33(6):1166-1182. doi: 10.1002/jbmr.3409. Epub 2018 Mar 23. J Bone Miner Res. 2018. PMID: 29461659 Free PMC article.
-
A disintegrin-like and metalloproteinase domain with thrombospondin type 1 motif 9 (ADAMTS9) regulates fibronectin fibrillogenesis and turnover.J Biol Chem. 2019 Jun 21;294(25):9924-9936. doi: 10.1074/jbc.RA118.006479. Epub 2019 May 13. J Biol Chem. 2019. PMID: 31085586 Free PMC article.
References
-
- Ashworth J. L., Kelly V., Wilson R., Shuttleworth C. A., Kielty C. M. Fibrillin assembly: dimer formation mediated by amino-terminal sequences. J. Cell Sci. 1999;112:3549–3558. - PubMed
-
- Balian G., Click E. M., Crouch E., Davidson J. M., Bornstein P. Isolation of a collagen-binding fragment from fibronectin and cold-insoluble globulin. J. Biol. Chem. 1979;254:1429–1432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

