Knockdown of p180 eliminates the terminal differentiation of a secretory cell line
- PMID: 19037105
- PMCID: PMC2626563
- DOI: 10.1091/mbc.e08-07-0682
Knockdown of p180 eliminates the terminal differentiation of a secretory cell line
Abstract
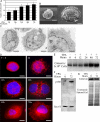
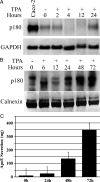
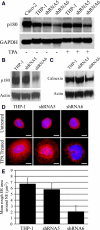
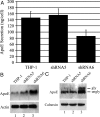
We have previously reported that the expression in yeast of an integral membrane protein (p180) of the endoplasmic reticulum (ER), isolated for its ability to mediate ribosome binding, is capable of inducing new membrane biogenesis and an increase in secretory capacity. To demonstrate that p180 is necessary and sufficient for terminal differentiation and acquisition of a secretory phenotype in mammalian cells, we studied the differentiation of a secretory cell line where p180 levels had been significantly reduced using RNAi technology and by transiently expressing p180 in nonsecretory cells. A human monocytic (THP-1) cell line, that can acquire macrophage-like properties, failed to proliferate rough ER when p180 levels were lowered. The Golgi compartment and the secretion of apolipoprotein E (Apo E) were dramatically affected in cells expressing reduced p180 levels. On the other hand, expression of p180 in a human embryonic kidney nonsecretory cell line (HEK293) showed a significant increase in proliferation of rough ER membranes and Golgi complexes. The results obtained from knockdown and overexpression experiments demonstrate that p180 is both necessary and sufficient to induce a secretory phenotype in mammalian cells. These findings support a central role for p180 in the terminal differentiation of secretory cells and tissues.
Figures



References
-
- Ambroziak J., Henry S. A. INO2 and INO4 gene products, positive regulators of phospholipid biosynthesis in Saccharomyces cerevisiae, form a complex that binds to the INO1 promoter. J. Biol. Chem. 1994;269:15344–15349. - PubMed
-
- Anderson D. J., Hetzer M. W. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat. Cell Biol. 2007;9:1160–1166. - PubMed
-
- Auwerx J. H., Deeb S., Brunzell J. D., Peng R., Chait A. Transcriptional activation of the lipoprotein lipase and apolipoprotein E genes accompanies differentiation in some human macrophage-like cell lines. Biochemistry. 1988;27:2651–2655. - PubMed
-
- Bai J. Z., Leung E., Holloway H., Krissansen G. W. Alternatively spliced forms of the P180 ribosome receptor differ in their ability to induce the proliferation of rough endoplasmic reticulum. Cell Biol. Int. 2007 doi: 10.1016/j.cellbi.2007.10.002. - DOI - PubMed
-
- Basheeruddin K., Rechtoris C., Mazzone T. Transcriptional and post-transcriptional control of apolipoprotein E gene expression in differentiating human monocytes. J. Biol. Chem. 1992;267:1219–1224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

