Disruption of desmosome assembly by monovalent human pemphigus vulgaris monoclonal antibodies
- PMID: 19037235
- PMCID: PMC2743719
- DOI: 10.1038/jid.2008.339
Disruption of desmosome assembly by monovalent human pemphigus vulgaris monoclonal antibodies
Abstract
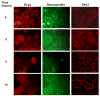
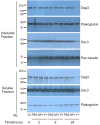
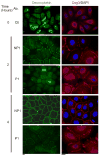
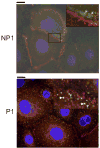
The intercellular interactions of the desmosomal cadherins, desmoglein and desmocollin, are required for epidermal cell adhesion. Pemphigus vulgaris (PV) is a potentially fatal autoimmune blistering disease characterized by autoantibodies against desmoglein (Dsg) 3. During calcium-induced desmosome assembly, treatment of primary human keratinocytes with pathogenic monovalent anti-Dsg3 mAbs produced from a PV patient causes a decrease of Dsg3 and desmoplakin but not desmocollin (Dsc) 3 in the Triton-insoluble fraction of cell lysates within 2 hours. Immunofluorescence and antibody ELISA studies suggest that pathogenic mAbs cause internalization of cell-surface Dsg3 but not Dsc3 through early endosomes. Electron microscopy demonstrated a lack of well-formed desmosomes in keratinocytes treated with pathogenic compared to nonpathogenic mAbs. In contrast, pathogenic mAbs caused late depletion of Dsg3 from preformed desmosomes at 24 hours, with effects on multiple desmosomal proteins including Dsc3 and plakoglobin. Together, these studies indicate that pathogenic PV mAbs specifically cause internalization of newly synthesized Dsg3 during desmosome assembly, correlating with their pathogenic activity. Monovalent human PV anti-Dsg mAbs reproduce the effects of polyclonal PV IgG on Dsg3 and will facilitate future studies to further dissect the cellular mechanisms for the loss of cell adhesion in pemphigus.
Conflict of interest statement
The authors state no conflict of interest.
Figures







References
-
- Amagai M, Karpati S, Klaus-Kovtun V, Udey MC, Stanley JR. The extracellular domain of pemphigus vulgaris antigen (desmoglein 3) mediates weak homophilic adhesion. J Invest Dermatol. 1994;102:402–408. - PubMed
-
- Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40:170. - PubMed
-
- Aoyama Y, Kitajima Y. Pemphigus vulgaris-IgG causes a rapid depletion of desmoglein 3 (Dsg3) from the Triton X-100 soluble pools, leading to the formation of Dsg3-depleted desmosomes in a human squamous carcinoma cell line, DJM-1 cells. J Invest Dermatol. 1999;112:67–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

