A yellow chlorophyll catabolite is a pigment of the fall colours
- PMID: 19037512
- PMCID: PMC2906697
- DOI: 10.1039/b813558d
A yellow chlorophyll catabolite is a pigment of the fall colours
Abstract
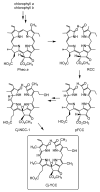
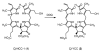
Here we describe the detection and identification of a yellow chlorophyll catabolite (Cj-YCC) in fresh extracts of senescent leaves of Cercidiphyllum japonicum. In addition, we report its partial synthesis by oxidation of Cj-NCC-1, the major (colourless) "nonfluorescent" chlorophyll catabolite (NCC) found in degreened leaves of C. japonicum. The spectroscopic analysis and structural characterization indicated Cj-YCC to be a simple dehydrogenation product of Cj-NCC-1 (by formal removal of a hydrogen atom at the C(20)- and C(1)-positions). Indeed, NCCs are easily oxidized and were first called "rusty pigments", as they had a tendency to turn brown upon storage on a dry silica gel plate. The yellow tetrapyrrole Cj-YCC may thus come about by oxidation of Cj-NCC-1 in the leaves. Its presence in the yellow leaves of a deciduous tree provides the first evidence for the contribution of a coloured chlorophyll catabolite to the fall colours.
Figures

References
-
- Bell CR, Lindsey AH. Fall Colors and Woodland Harvests. Laurel Hill Press; Chapel Hill, USA: 1990.
-
- Brown SB, Houghton JD, Hendry GAF. In: Chlorophylls. Scheer H, editor. CRP-Press; Boca Raton, USA: 1991. pp. 465–489.
-
- Matile P. Senescence in Plants and Its Importance for Nitrogen-Metabolism. Chimia. 1987;41:376–381.
-
- Kräutler B, Jaun B, Bortlik K, Schellenberg M, Matile P. On the Enigma of Chlorophyll Degradation - the Constitution of a Secoporphinoid Catabolite. Angew. Chem., Int. Ed. 1991;30:1315–1318.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

