An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production
- PMID: 19038867
- PMCID: PMC2720268
- DOI: 10.1161/CIRCRESAHA.108.178467
An ezrin/calpain/PI3K/AMPK/eNOSs1179 signaling cascade mediating VEGF-dependent endothelial nitric oxide production
Abstract
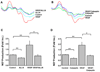
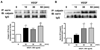
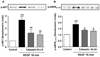
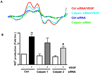
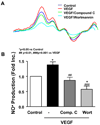
Calpain was recently reported to mediate vascular endothelial growth factor (VEGF)-induced angiogenesis. In the present study, we investigated detailed molecular mechanisms. VEGF (100 ng/mL) induced a marked increase in endothelial cell production of NO(*), specifically detected by electron spin resonance. This response was abolished by inhibition of calpain with N-acetyl-leucyl-leucyl-norleucinal (ALLN) or Calpeptin. Both also diminished membrane-specific calpain activation by VEGF, which was intriguingly attenuated by silencing ezrin with RNA interference. A rapid membrane colocalization of calpain and ezrin occurred as short as 10 minutes after VEGF stimulation. AKT, AMP-dependent kinase (AMPK), and endothelial nitric oxide synthase (eNOS)(s1179) phosphorylations in VEGF-stimulated endothelial cells were markedly enhanced, which were however significantly attenuated by either ALLN, Calpeptin, or ezrin small interfering RNA, as well as by Wortmannin or compound C (respectively for phosphatidylinositol 3-kinase [PI3K] or AMPK). The latter 3 also abolished VEGF induction of NO(*). These data indicate that AMPK and AKT are both downstream of PI3K and that AKT activation is partially dependent on AMPK. The interrelationship between AMPK and AKT, although known to be individually important in mediating VEGF activation of eNOS, is clearly characterized. Furthermore, AMPK/AKT/eNOS(s1179) was found downstream of a calpain/ezrin membrane interaction. These data no doubt provide new insights into the long mystified signaling gap between VEGF receptors and PI3K/AKT or AMPK-dependent eNOS activation. In view of the well-established significance of VEGF-dependent angiogenesis, these findings might have broad and important implications in cardiovascular pathophysiology.
Figures










References
-
- Khorchid A, Ikura M. How calpain is activated by calcium. Nat Struct Biol. 2002;9:239–241. - PubMed
-
- Tompa P, Emori Y, Sorimachi H, Suzuki K, Friedrich P. Domain III of calpain is a ca2+regulated phospholipid-binding domain. Biochem Biophys Res Commun. 2001;280:1333–1339. - PubMed
-
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. - PubMed
-
- Kulkarni S, Saido TC, Suzuki K, Fox JE. Calpain mediates integrin-induced signaling at a point upstream of Rho family members. J Biol Chem. 1999;274:21265–21275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

