Stromal gene signatures in large-B-cell lymphomas
- PMID: 19038878
- PMCID: PMC9103713
- DOI: 10.1056/NEJMoa0802885
Stromal gene signatures in large-B-cell lymphomas
Abstract
Background: The addition of rituximab to combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), or R-CHOP, has significantly improved the survival of patients with diffuse large-B-cell lymphoma. Whether gene-expression signatures correlate with survival after treatment of diffuse large-B-cell lymphoma is unclear.
Methods: We profiled gene expression in pretreatment biopsy specimens from 181 patients with diffuse large-B-cell lymphoma who received CHOP and 233 patients with this disease who received R-CHOP. A multivariate gene-expression-based survival-predictor model derived from a training group was tested in a validation group.
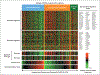
Results: A multivariate model created from three gene-expression signatures--termed "germinal-center B-cell," "stromal-1," and "stromal-2"--predicted survival both in patients who received CHOP and patients who received R-CHOP. The prognostically favorable stromal-1 signature reflected extracellular-matrix deposition and histiocytic infiltration. By contrast, the prognostically unfavorable stromal-2 signature reflected tumor blood-vessel density.
Conclusions: Survival after treatment of diffuse large-B-cell lymphoma is influenced by differences in immune cells, fibrosis, and angiogenesis in the tumor microenvironment.
2008 Massachusetts Medical Society
Figures

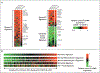

Comment in
-
Microenvironmental protection in diffuse large-B-cell lymphoma.N Engl J Med. 2008 Nov 27;359(22):2379-81. doi: 10.1056/NEJMe0808409. N Engl J Med. 2008. PMID: 19038884 No abstract available.
-
Molecular outcome prediction in diffuse large-B-cell lymphoma.N Engl J Med. 2009 Jun 25;360(26):2794-5. doi: 10.1056/NEJMc0902616. N Engl J Med. 2009. PMID: 19553658 No abstract available.
References
-
- Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 1993;328:1002–6. - PubMed
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–42. - PubMed
-
- Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–47. - PubMed
-
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–11. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
