Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion
- PMID: 19039100
- PMCID: PMC2651457
- DOI: 10.1093/jxb/ern290
Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion
Abstract
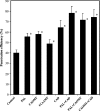
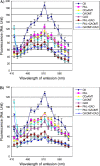
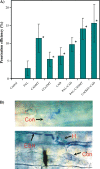
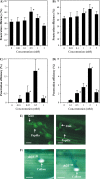
Cell wall apposition (CWA) formation is one of the first lines of defence used by plants to halt invading fungi such as powdery mildew. Lignin is a complex polymer of hydroxylated and methoxylated phenylpropane units (monolignols) and lignification renders the cell wall more resistant to pathogen attack. The role of monolignol biosynthesis in CWA-mediated defence against powdery mildew penetration into cereals is demonstrated here using RNA interference (RNAi)-mediated gene silencing and enzyme-specific inhibitors. Thirteen cDNAs representing eight genes involved in monolignol biosynthesis were cloned from an expression sequence tag (EST) library derived from the epidermis of diploid wheat (Triticum monococcum) infected with Blumeria graminis f. sp. tritici (Bgt). Differential expression patterns were found for these genes in susceptible and resistant plants after infection. Transcripts of phenylalanine ammonia lyase (PAL), caffeic acid O-methyltransferase (CAOMT), ferulic acid hydroxylase (FAH), caffeoyl-CoA O-methyltransferase (CCoAMT), and cinnamyl alcohol dehydrogenase (CAD) were accumulated, particularly in the epidermis. RNAi-mediated transient gene silencing in the epidermis led to a higher penetration efficiency of Bgt than in the controls. Gene silencing also compromised penetration resistance to varying degrees with different genes against an inappropriate pathogen, B. graminis f. sp. hordei (Bgh). Co-silencing led to greater penetration of Bgt or Bgh than when the genes were silenced separately. Fluorescence emission spectra analyses revealed that gene silencing hampered host autofluorescence response at fungal contact sites. These results illustrate that monolignol biosynthesis is critically important for host defence against both appropriate and inappropriate pathogen invasion in wheat.
Figures



Comment in
-
Cell wall appositions: the first line of defence.J Exp Bot. 2009;60(2):351-2. doi: 10.1093/jxb/erp001. Epub 2009 Feb 9. J Exp Bot. 2009. PMID: 19204034 No abstract available.
-
Role of lignification in plant defense.Plant Signal Behav. 2009 Feb;4(2):158-9. doi: 10.4161/psb.4.2.7688. Plant Signal Behav. 2009. PMID: 19649200 Free PMC article.
References
-
- Beardmore J, Ride JP, Granger JW. Cellular lignifications a factor in the hypersensitive resistance of wheat to stem rust. Physiological and Molecular Plant Pathology. 1983;22:209–220.
-
- Bhuiyan NH, Liu W, Liu G, Selvaraj G, Wei Y, King J. Transcriptional regulation of genes involved in the pathways of biosynthesis and supply of methyl units in response to powdery mildew attack and abiotic stresses in wheat. Plant Molecular Biology. 2007;64:305–318. - PubMed
-
- Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annual Review of Plant Biology. 2003;54:519–546. - PubMed
-
- Boudet AM, Kajita S, Grima-Pettenati J, Goffner D. Biochemistry and molecular biology of lignification. New Phytologist. 1995;129:203–236. - PubMed
-
- Boyd LA, Smith PH, Foster EM, Brown JKM. The effects of allelic variation at the Mla resistance locus in barley on the early development of Erysiphe graminis f.sp. hordei and host responses. The Plant Journal. 1995;7:959–968.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

