PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release
- PMID: 19039139
- PMCID: PMC2723733
- DOI: 10.1126/science.1164571
PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release
Abstract
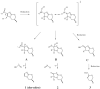
Bicyclic nitroimidazoles, including PA-824, are exciting candidates for the treatment of tuberculosis. These prodrugs require intracellular activation for their biological function. We found that Rv3547 is a deazaflavin-dependent nitroreductase (Ddn) that converts PA-824 into three primary metabolites; the major one is the corresponding des-nitroimidazole (des-nitro). When derivatives of PA-824 were used, the amount of des-nitro metabolite formed was highly correlated with anaerobic killing of Mycobacterium tuberculosis (Mtb). Des-nitro metabolite formation generated reactive nitrogen species, including nitric oxide (NO), which are the major effectors of the anaerobic activity of these compounds. Furthermore, NO scavengers protected the bacilli from the lethal effects of the drug. Thus, these compounds may act as intracellular NO donors and could augment a killing mechanism intrinsic to the innate immune system.
Figures
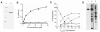
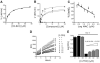
Comment in
-
Microbiology. An antibiotic mimics immunity.Science. 2008 Nov 28;322(5906):1337-8. doi: 10.1126/science.1167452. Science. 2008. PMID: 19039126 No abstract available.
References
-
- Matteelli A, et al. Expert Rev. Anti Infect. Ther. 2007;5:857. - PubMed
-
- Barry CE, 3rd, Boshoff HI, Dowd CS. Curr. Pharm. Des. 2004;10:3239. - PubMed
-
- StopTB Working Group New Drugs. 2008. www.stoptb.org/wg/ new_drugs/assets/documents/2007GlobalPipeline.pdf.
-
- Stover CK, et al. Nature. 2000;405:962. - PubMed
-
- Boshoff HI, Barry CE., 3rd Nat. Rev. Microbiol. 2005;3:70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

