Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases
- PMID: 19040558
- PMCID: PMC6493995
- DOI: 10.1111/j.1755-5949.2008.00060.x
Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases
Abstract
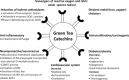
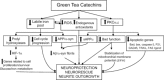
Current therapeutic approaches for Alzheimer and Parkinson disease (AD and PD, respectively) are merely symptomatic, intended for the treatment of symptoms, but offer only partial benefit, without any disease-modifying activity. Novel promising strategies suggest the use of antiinflammatory drugs, antioxidants, iron-complexing molecules, neurotrophic factor delivery, inhibitors of the amyloid precursor protein (APP)-processing secretases, gamma and beta (that generate the amyloid-beta peptides, Abeta), anti-Abeta aggregation molecules, the interference with lipid cholesterol metabolism and naturally occurring plant flavonoids to potentially reverse the course of the diseases. Human epidemiological and new animal data suggest that tea drinking may decrease the incidence of dementia, AD, and PD. In particular, its main catechin polyphenol constituent (-)-epigallocatechin-3-gallate (EGCG) has been shown to exert neuroprotective/neurorescue activities in a wide array of cellular and animal models of neurological disorders. In the current article, we review the literature on the impact of the multimodal activities of green tea polyphenols and their neuroprotective effect on AD and PD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gutman RL RB. Rediscovering tea: An exploration of the scientific literature. HerbalGram 1996;37:33–48.
-
- Wang ZY, Huang MT, Lou YR, Xie JG, Reuhl KR, Newmark HL, Ho CT, Yang CS, Conney AH. Inhibitory effects of black tea, green tea, decaffeinated black tea, and decaffeinated green tea on ultraviolet B light‐induced skin carcinogenesis in 7,12‐dimethylbenz[a]anthracene‐initiated SKH‐1 mice. Cancer Res 1994;54:3428–3455. - PubMed
-
- Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst 1993;85:1038–1049. - PubMed
-
- Khokhar S, Magnusdottir SG. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United kingdom. J Agric Food Chem 2002;50:565–570. - PubMed
-
- Higdon JV, Frei B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 2003;43:89–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

