In vivo characterization of transplanted human embryonic stem cell-derived pancreatic endocrine islet cells
- PMID: 19040565
- PMCID: PMC6495805
- DOI: 10.1111/j.1365-2184.2008.00564.x
In vivo characterization of transplanted human embryonic stem cell-derived pancreatic endocrine islet cells
Abstract
Objectives: Islet-like clusters (ILCs), differentiated from human embryonic stem cells (hESCs), were characterized both before and after transplantation under the kidney capsule of streptozotocin-induced diabetic immuno-incompetent mice.
Materials and methods: Multiple independent ILC preparations (n = 8) were characterized by immunohistochemistry, flow cytometry and cell insulin content, with six preparations transplanted into diabetic mice (n = 42), compared to controls, which were transplanted with either a human fibroblast cell line or undifferentiated hESCs (n = 28).
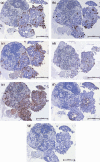
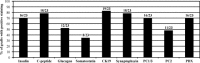
Results: Prior to transplantation, ILCs were immunoreactive for the islet hormones insulin, C-peptide and glucagon, and for the ductal epithelial marker cytokeratin-19. ILCs also had cellular insulin contents similar to or higher than human foetal islets. Expression of islet and pancreas-specific cell markers was maintained for 70 days post-transplantation. The mean survival of recipients was increased by transplanted ILCs as compared to transplanted human fibroblast cells (P < 0.0001), or undifferentiated hESCs (P < 0.042). Graft function was confirmed by secretion of human C-peptide in response to an oral bolus of glucose.
Conclusions: hESC-derived ILC grafts continued to contain cells that were positive for islet endocrine hormones and were shown to be functional by their ability to secrete human C-peptide. Further enrichment and maturation of ILCs could lead to generation of a sufficient source of insulin-producing cells for transplantation into patients with type 1 diabetes.
Figures




References
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz‐Eldor J, Thomson JA (2000) Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 227, 271–278. - PubMed
-
- Assady S, Maor G, Amit M, Itskovitz‐Eldor J, Skorecki KL, Tzukerman M (2001) Insulin production by human embryonic stem cells. Diabetes 50, 1691–1697. - PubMed
-
- Baharvand H, Jafary H, Massumi M, Ashtiani SK (2006) Generation of insulin‐secreting cells from human embryonic stem cells. Dev. Growth Differ. 48, 323–332. - PubMed
-
- Blyszczuk P, Asbrand C, Rozzo A, Kania G, St Onge L, Rupnik M, Wobus AM (2004) Embryonic stem cells differentiate into insulin‐producing cells without selection of nestin‐expressing cells. Int. J. Dev. Biol. 48, 1095–1104. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

