Role of alpha7-nicotinic acetylcholine receptor in human non-small cell lung cancer proliferation
- PMID: 19040571
- PMCID: PMC9531952
- DOI: 10.1111/j.1365-2184.2008.00566.x
Role of alpha7-nicotinic acetylcholine receptor in human non-small cell lung cancer proliferation
Erratum in
- Cell Prolif. 2009 Aug;42(4):568
- Cell Prolif. 2009 Feb;42(1):122
Retraction in
-
Retraction Statement: Role of α7-nicotinic acetylcholine receptor in human non-small cell lung cancer proliferation.Cell Prolif. 2017 Dec;50(6):e12379. doi: 10.1111/cpr.12379. Epub 2017 Aug 29. Cell Prolif. 2017. PMID: 36230005 Free PMC article.
Abstract
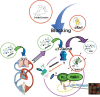
Objectives: Lung cancer is the most common cause of cancer death in the world. Cigarette smoking represents the major risk factor. Nicotine, an active component of cigarettes, can induce cell proliferation, angiogenesis and apoptosis resistance. All these events are mediated through the nicotinic acetylcholine receptor (nAChR) expressed on lung cancer cells. We speculate that new insights into the pathophysiological roles of nAChR may lead to new therapeutic avenues to reduce non-small cell lung cancer (NSCLC) tumour growth.
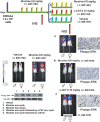
Materials and methods: Human samples of NSCLC, cell lines and mouse models were utilized in Western blotting, reverse transcriptase polymerase chain reaction and apoptosis studies.
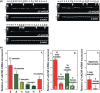
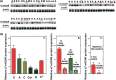
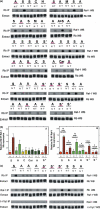
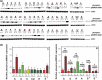
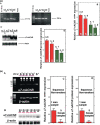
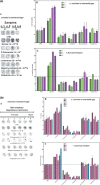
Results: Human NSCLC tissues expressed alpha7-nAChR. This expression was higher in smoking patients with squamous carcinomas than those with adenocarcinomas and in male smoking patients than in females. All the data support the hypothesis that major expression of alpha7-nAChR is related to major activation of the Rb-Raf-1/phospho-ERK/phospho-p90RSK pathway. alpha7-nAChR antagonists, via mitochondria associated apoptosis, inhibited proliferation of human NSCLC primary and established cells. Nicotine stimulates tumour growth in a murine model, A549 cells orthotopically grafted. The effects of nicotine were associated with increases in phospho-ERK in tumours. Proliferation effects of nicotine could be blocked by inhibition of alpha7-nAChR by the high affinity ligand alpha-cobratoxin.
Conclusion: These results showed that alpha7-nAChR plays an important role in NSCLC cell growth and tumour progression as well as in cell death.
Figures



References
-
- Antil‐Delbeke S, Gaillard C, Tamiya T (2000) Molecular determinants by which a long chain toxin from snake venom interacts with the neuronal alpha7‐nicotinic acetylcholine receptor. J. Biol. Chem. 275, 29594–29601. - PubMed
-
- Arredondo J, Chernyavsky AI, Grando SA (2006a) Nicotinic receptors mediate tumorigenic action of tobacco‐derived nitrosamines on immortalized oral epithelial cells. Cancer Biol. Ther. 5, 511–517. - PubMed
-
- Battersby AR, Hodson HF (1968) Alkaloids of calabash curare and Strychnos species. In: Manske RHF, ed. The Alkaloids, Chemistry and Physiology, Vol. 11. Academic Press, New York, pp. 189–204.
-
- Boffetta P, Agudo A, Ahrens W, Benhamou E, Benhamou S, Darby SC, Ferro G, Fortes C, Gonzalez CA, Jockel KH, Krauss M, Kreienbrock L, Kreuzer M, Mendes A, Merletti F, Nyberg F, Pershagen G, Pohlabeln H, Riboli E, Schmid G, Simonato L, Tredaniel J, Whitley E, Wichmann HE, Winck C, Zambon P, Saracci R (1998) Multicenter case control study of exposure to environmental tobacco smoke and lung cancer in Europe. J. Natl Cancer Inst. 90, 1440–1450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

