Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study
- PMID: 19040615
- PMCID: PMC2675245
- DOI: 10.1111/j.1365-2249.2008.03814.x
Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study
Abstract
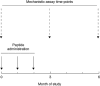
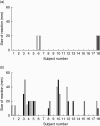
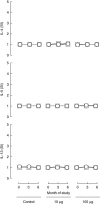
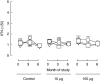
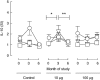
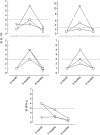
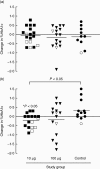
Immunotherapeutic strategies under consideration for type 1 diabetes include modification of the autoimmune response through antigen-specific routes. Administration of short peptides representing T cell epitopes targeted by patients with the disease represents one approach. This study evaluated safety and mechanistic outcomes during first-in-man intradermal administration of a human leucocyte antigen-DR4 (HLA-DR4)-restricted peptide epitope of proinsulin (C19-A3). This randomized, open-label study assessed two major theoretical risks of peptide immunotherapy, namely induction of allergic hypersensitivity and exacerbation of the proinflammatory autoimmune response, using clinical assessment and mechanistic assays in vitro. Patients with long-standing type 1 diabetes and HLA-DRB1*0401 genotype received 30 microg (n = 18) or 300 microg (n = 18) of peptide in three equal doses at 0, 1 and 2 months or no intervention (n = 12). Proinsulin peptide immunotherapy in the dosing regimen used is well tolerated and free from risk of systemic hypersensitivity and induction/reactivation of proinsulin-specific, proinflammatory T cells. Peptide-specific T cells secreting the immune suppressive cytokine interleukin (IL)-10 were observed at month 3 in four of 18 patients in the low-dose group (versus one of 12 in the control group; P = not significant). Mean IL-10 response to peptide in the low-dose group increased between 0 and 3 months (P = 0.05 after stimulation with 5 microM peptide in vitro) and then declined to baseline levels between 3 and 6 months (P = 0.01 at 10 microM peptide in vitro). These studies pave the way for future investigations in new-onset patients designed to examine whether proinsulin peptide immunotherapy has beneficial effects on markers of T cell autoimmunity and preservation of beta cell mass.
Figures
References
-
- Bougneres PF, Landais P, Boisson C, et al. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39:1264–72. - PubMed
-
- Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. - PubMed
-
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. - PubMed
-
- Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immun. 2005;23:115–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

