Polarity and differential inheritance--universal attributes of life?
- PMID: 19041746
- PMCID: PMC2844324
- DOI: 10.1016/j.cell.2008.11.006
Polarity and differential inheritance--universal attributes of life?
Abstract
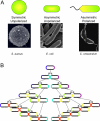
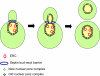
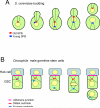
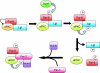
When and why did cell polarization arise? Recent work in bacteria and yeast suggests that polarization may have evolved to restrict senescence to one daughter during division by enabling the differential segregation of damaged material. In more complex organisms, polarity functions have diversified to permit the differential inheritance of centrosomes, RNAs, proteins, and membranes, which is essential for the generation of diverse cell types from stem cells and for morphogenesis.
Figures

References
-
- Ackermann M, Stearns SC, Jenal U. Senescence in a bacterium with asymmetric division. Science. 2003;300:1920. - PubMed
-
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. - PubMed
-
- Barker MG, Walmsley RM. Replicative ageing in the fission yeast Schizosaccharomyces pombe. Yeast. 1999;15:1511–1518. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

