N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake
- PMID: 19041747
- PMCID: PMC2643061
- DOI: 10.1016/j.cell.2008.10.043
N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake
Abstract
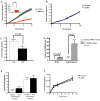
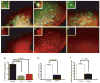
N-acylphosphatidylethanolamines (NAPEs) are a relatively abundant group of plasma lipids of unknown physiological significance. Here, we show that NAPEs are secreted into circulation from the small intestine in response to ingested fat and that systemic administration of the most abundant circulating NAPE, at physiologic doses, decreases food intake in rats without causing conditioned taste aversion. Furthermore, (14)C-radiolabeled NAPE enters the brain and is particularly concentrated in the hypothalamus, and intracerebroventricular infusions of nanomolar amounts of NAPE reduce food intake, collectively suggesting that its effects may be mediated through direct interactions with the central nervous system. Finally, chronic NAPE infusion results in a reduction of both food intake and body weight, suggesting that NAPE and long-acting NAPE analogs may be novel therapeutic targets for the treatment of obesity.
Figures





References
-
- Abizaid A, Gao Q, et al. Thoughts for food: brain mechanisms and peripheral energy balance. Neuron. 2006;51(6):691–702. - PubMed
-
- Cone RD, Cowley MA, et al. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–7. - PubMed
-
- Cota D, Proulx K, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

