Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma
- PMID: 19041750
- PMCID: PMC3015046
- DOI: 10.1016/j.cell.2008.09.045
Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma
Abstract
Loss of cell polarity proteins such as Scribble induces neoplasia in Drosophila by promoting uncontrolled proliferation. In mammals, the role that polarity proteins play during tumorigenesis is not well understood. Here, we demonstrate that depletion of Scribble in mammary epithelia disrupts cell polarity, blocks three-dimensional morphogenesis, inhibits apoptosis, and induces dysplasia in vivo that progress to tumors after long latency. Loss of Scribble cooperates with oncogenes such as c-myc to transform epithelial cells and induce tumors in vivo by blocking activation of an apoptosis pathway. Like depletion, mislocalization of Scribble from cell-cell junction was sufficient to promote cell transformation. Interestingly, spontaneous mammary tumors in mice and humans possess both downregulated and mislocalized Scribble. Thus, we demonstrate that scribble inhibits breast cancer formation and that deregulation of polarity pathways promotes dysplastic and neoplastic growth in mammals by disrupting morphogenesis and inhibiting cell death.
Figures
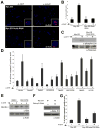

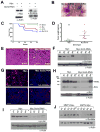
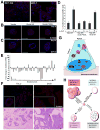
Similar articles
-
Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with basal breast cancer.Cancer Res. 2014 Jun 1;74(11):3180-94. doi: 10.1158/0008-5472.CAN-13-3415. Epub 2014 Mar 24. Cancer Res. 2014. PMID: 24662921 Free PMC article.
-
Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling.Oncogene. 2008 Oct 9;27(46):5988-6001. doi: 10.1038/onc.2008.219. Epub 2008 Jul 21. Oncogene. 2008. PMID: 18641685
-
Scribble modulates the MAPK/Fra1 pathway to disrupt luminal and ductal integrity and suppress tumour formation in the mammary gland.PLoS Genet. 2014 May 22;10(5):e1004323. doi: 10.1371/journal.pgen.1004323. eCollection 2014 May. PLoS Genet. 2014. PMID: 24852022 Free PMC article.
-
The Case of the Scribble Polarity Module in Asymmetric Neuroblast Division in Development and Tumorigenesis.Int J Mol Sci. 2020 Apr 20;21(8):2865. doi: 10.3390/ijms21082865. Int J Mol Sci. 2020. PMID: 32325951 Free PMC article. Review.
-
The Scribble-Dlg-Lgl polarity module in development and cancer: from flies to man.Essays Biochem. 2012;53:141-68. doi: 10.1042/bse0530141. Essays Biochem. 2012. PMID: 22928514 Review.
Cited by
-
Polarity proteins as regulators of cell junction complexes: implications for breast cancer.Pharmacol Ther. 2013 Jun;138(3):418-27. doi: 10.1016/j.pharmthera.2013.02.004. Epub 2013 Feb 28. Pharmacol Ther. 2013. PMID: 23458609 Free PMC article. Review.
-
Cell polarity changes in cancer initiation and progression.J Cell Biol. 2024 Jan 1;223(1):e202308069. doi: 10.1083/jcb.202308069. Epub 2023 Dec 13. J Cell Biol. 2024. PMID: 38091012 Free PMC article. Review.
-
An interaction between Scribble and the NADPH oxidase complex controls M1 macrophage polarization and function.Nat Cell Biol. 2016 Nov;18(11):1244-1252. doi: 10.1038/ncb3413. Epub 2016 Oct 3. Nat Cell Biol. 2016. PMID: 27694890
-
Scribble acts as an oncogene in Eμ-myc-driven lymphoma.Oncogene. 2016 Mar 3;35(9):1193-7. doi: 10.1038/onc.2015.167. Epub 2015 May 18. Oncogene. 2016. PMID: 25982280
-
K-Ras and B-Raf oncogenes inhibit colon epithelial polarity establishment through up-regulation of c-myc.J Cell Biol. 2012 Jul 23;198(2):185-94. doi: 10.1083/jcb.201202108. J Cell Biol. 2012. PMID: 22826122 Free PMC article.
References
-
- Aguilar-Cordova E, Strange R, Young LJ, Billy HT, Gumerlock PH, Cardiff RD. Viral Ha-ras mediated mammary tumor progression. Oncogene. 1991;6:1601–1607. - PubMed
-
- Allred CA, Hilsenback SG, Mohsin SK. Biological features of human premaligant breast disease. In: Harris J, Lippman ME, Morrow M, Osborne K, editors. Diseases of the Breast. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 507–520.
-
- Allred DC, Mohsin SK, Fuqua SA. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer. 2001;8:47–61. - PubMed
-
- Amundadottir LT, Johnson MD, Merlino G, Smith GH, Dickson RB. Synergistic interaction of transforming growth factor alpha and c-myc in mouse mammary and salivary gland tumorigenesis. Cell Growth Differ. 1995;6:737–748. - PubMed
-
- Amundadottir LT, Nass SJ, Berchem GJ, Johnson MD, Dickson RB. Cooperation of TGF alpha and c-Myc in mouse mammary tumorigenesis: coordinated stimulation of growth and suppression of apoptosis. Oncogene. 1996;13:757–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

