Fate tracing reveals the endothelial origin of hematopoietic stem cells
- PMID: 19041779
- PMCID: PMC2631552
- DOI: 10.1016/j.stem.2008.09.018
Fate tracing reveals the endothelial origin of hematopoietic stem cells
Abstract
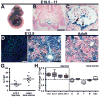
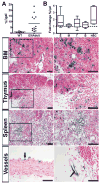
Hematopoietic stem cells (HSCs) originate within the aortic-gonado-mesonephros (AGM) region of the midgestation embryo, but the cell type responsible for their emergence is unknown since critical hematopoietic factors are expressed in both the AGM endothelium and its underlying mesenchyme. Here we employ a temporally restricted genetic tracing strategy to selectively label the endothelium, and separately its underlying mesenchyme, during AGM development. Lineage tracing endothelium, via an inducible VE-cadherin Cre line, reveals that the endothelium is capable of HSC emergence. The endothelial progeny migrate to the fetal liver, and later to the bone marrow, and are capable of expansion, self-renewal, and multilineage hematopoietic differentiation. HSC capacity is exclusively endothelial, as ex vivo analyses demonstrate lack of VE-cadherin Cre induction in circulating and fetal liver hematopoietic populations. Moreover, AGM mesenchyme, as selectively traced via a myocardin Cre line, is incapable of hematopoiesis. Our genetic tracing strategy therefore reveals an endothelial origin of HSCs.
Figures
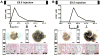




References
-
- Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235(3):759–767. - PubMed
-
- Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development (Cambridge, England) 2003;130(22):5437–5444. - PubMed
-
- Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin I, Cumano A. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(1):134–139. - PMC - PubMed
-
- Bollerot K, Pouget C, Jaffredo T. The embryonic origins of hematopoietic stem cells: a tale of hemangioblast and hemogenic endothelium. Apmis. 2005;113(1112):790–803. - PubMed
-
- Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87(2):630–641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

