TNF-induced mitochondrial damage: a link between mitochondrial complex I activity and left ventricular dysfunction
- PMID: 19041937
- PMCID: PMC2735225
- DOI: 10.1016/j.freeradbiomed.2008.10.049
TNF-induced mitochondrial damage: a link between mitochondrial complex I activity and left ventricular dysfunction
Abstract
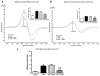
Mitochondrial damage is implicated in the progression of cardiac disease. Considerable evidence suggests that proinflammatory cytokines induce oxidative stress and contribute to cardiac dysfunction. This study was conducted to determine whether a TNF-induced increase in superoxide (O(2)(*)(-)) contributes to mitochondrial damage in the left ventricle (LV) by impairing respiratory complex I activity. We employed an electron paramagnetic resonance (EPR) method to measure O(2)(*)(-) and oxygen consumption in mitochondrial respiratory complexes, using an oxygen label. Adult male Sprague-Dawley rats were divided into four groups: control, TNF treatment (ip), TNF+ apocynin (APO; 200 micromol/kg bw, orally), and TNF+ Tempol (Temp; 300 micromol/kg bw, orally). TNF was injected daily for 5 days. Rats were sacrificed, LV tissue was collected, and mitochondria were isolated for EPR studies. Total LV ROS production was significantly higher in TNF animals than in controls; APO or Temp treatment ameliorated TNF-induced LV ROS production. Total mitochondrial ROS production was significantly higher in the TNF and TNF+ APO groups than in the control and TNF+ Temp groups. These findings suggest that TNF alters the cellular redox state, reduces the expression of four complex I subunits by increasing mitochondrial O(2)(*)(-) production and depleting ATP synthesis, and decreases oxygen consumption, thereby resulting in mitochondrial damage and leading to LV dysfunction.
Figures





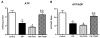
Similar articles
-
Interaction of TNF with angiotensin II contributes to mitochondrial oxidative stress and cardiac damage in rats.PLoS One. 2012;7(10):e46568. doi: 10.1371/journal.pone.0046568. Epub 2012 Oct 9. PLoS One. 2012. PMID: 23056347 Free PMC article.
-
TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol.Am J Physiol Heart Circ Physiol. 2007 Nov;293(5):H2726-37. doi: 10.1152/ajpheart.00376.2007. Epub 2007 Aug 3. Am J Physiol Heart Circ Physiol. 2007. PMID: 17675574
-
Mitochondria and NADPH oxidases are the major sources of TNF-α/cycloheximide-induced oxidative stress in murine intestinal epithelial MODE-K cells.Cell Signal. 2015 Jun;27(6):1141-58. doi: 10.1016/j.cellsig.2015.02.019. Epub 2015 Feb 26. Cell Signal. 2015. PMID: 25725292
-
Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers.J Neurosci Res. 2007 Aug 1;85(10):2216-23. doi: 10.1002/jnr.21360. J Neurosci Res. 2007. PMID: 17510982
-
GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect.Am J Physiol. 1997 Jul;273(1 Pt 1):G7-17. doi: 10.1152/ajpgi.1997.273.1.G7. Am J Physiol. 1997. PMID: 9252504 Review.
Cited by
-
COVID-19: Proposing a Ketone-Based Metabolic Therapy as a Treatment to Blunt the Cytokine Storm.Oxid Med Cell Longev. 2020 Sep 9;2020:6401341. doi: 10.1155/2020/6401341. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33014275 Free PMC article. Review.
-
Interaction of TNF with angiotensin II contributes to mitochondrial oxidative stress and cardiac damage in rats.PLoS One. 2012;7(10):e46568. doi: 10.1371/journal.pone.0046568. Epub 2012 Oct 9. PLoS One. 2012. PMID: 23056347 Free PMC article.
-
NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes.Cardiovasc Res. 2010 Feb 1;85(3):473-83. doi: 10.1093/cvr/cvp305. Epub 2009 Sep 3. Cardiovasc Res. 2010. PMID: 19729361 Free PMC article.
-
The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase.Biomaterials. 2010 Jul;31(19):5218-26. doi: 10.1016/j.biomaterials.2010.03.026. Epub 2010 Apr 7. Biomaterials. 2010. PMID: 20378166 Free PMC article.
-
Mitochondrial function in hypoxic ischemic injury and influence of aging.Prog Neurobiol. 2017 Oct;157:92-116. doi: 10.1016/j.pneurobio.2016.06.006. Epub 2016 Jun 16. Prog Neurobiol. 2017. PMID: 27321753 Free PMC article. Review.
References
-
- Sekiguchi K, Li X, Coker M, Flesch M, Barger PM, Sivasubramanian N, Mann DL. Cross-regulation between the renin–angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc Res. 2004;63:433–442. - PubMed
-
- Francis J, Zhang ZH, Weiss RM, Felder RB. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H791–797. - PubMed
-
- Pignatelli P, Cangemi R, Celestini A, Carnevale R, Polimeni L, Martini A, Ferro D, Loffredo L, Violi F. Tumour necrosis factor α upregulates platelet CD40 L in patients with heart failure. Cardiovasc Res. 2008;78:515–522. - PubMed
-
- Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, Felder RB. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor kappaB. Hypertension. 2007;49:511–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

