A novel adhesion molecule in human breast cancer cells: voltage-gated Na+ channel beta1 subunit
- PMID: 19041953
- PMCID: PMC2678854
- DOI: 10.1016/j.biocel.2008.11.001
A novel adhesion molecule in human breast cancer cells: voltage-gated Na+ channel beta1 subunit
Abstract
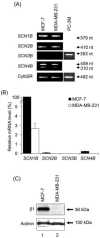
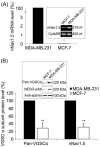
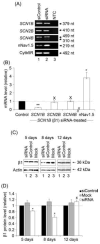
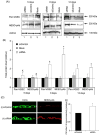
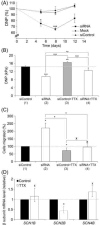
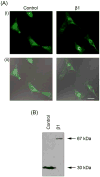
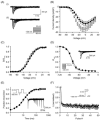
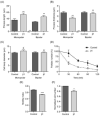
Voltage-gated Na(+) channels (VGSCs), predominantly the 'neonatal' splice form of Na(v)1.5 (nNa(v)1.5), are upregulated in metastatic breast cancer (BCa) and potentiate metastatic cell behaviours. VGSCs comprise one pore-forming alpha subunit and one or more beta subunits. The latter modulate VGSC expression and gating, and can function as cell adhesion molecules of the immunoglobulin superfamily. The aims of this study were (1) to determine which beta subunits were expressed in weakly metastatic MCF-7 and strongly metastatic MDA-MB-231 human BCa cells, and (2) to investigate the possible role of beta subunits in adhesion and migration. In both cell lines, the beta subunit mRNA expression profile was SCN1B (encoding beta1)>>SCN4B (encoding beta4)>SCN2B (encoding beta2); SCN3B (encoding beta3) was not detected. MCF-7 cells had much higher levels of all beta subunit mRNAs than MDA-MB-231 cells, and beta1 mRNA was the most abundant. Similarly, beta1 protein was strongly expressed in MCF-7 and barely detectable in MDA-MB-231 cells. In MCF-7 cells transfected with siRNA targeting beta1, adhesion was reduced by 35%, while migration was increased by 121%. The increase in migration was reversed by tetrodotoxin (TTX). In addition, levels of nNa(v)1.5 mRNA and protein were increased following beta1 down-regulation. Stable expression of beta1 in MDA-MB-231 cells increased functional VGSC activity, process length and adhesion, and reduced lateral motility and proliferation. We conclude that beta1 is a novel cell adhesion molecule in BCa cells and can control VGSC (nNa(v)1.5) expression and, concomitantly, cellular migration.
Figures
References
-
- Adachi K, Toyota M, Sasaki Y, Yamashita T, Ishida S, Ohe-Toyota M, Maruyama R, Hinoda Y, Saito T, Imai K, Kudo R, Tokino T. Identification of SCN3B as a novel p53-inducible proapoptotic gene. Oncogene. 2004;23:7791–7798. - PubMed
-
- Allen DH, Lepple-Wienhues A, Cahalan MD. Ion channel phenotype of melanoma cell lines. J Membr Biol. 1997;155:27–34. - PubMed
-
- An RH, Wang XL, Kerem B, Benhorin J, Medina A, Goldmit M, Kass RS. Novel LQT-3 mutation affects Na+ channel activity through interactions between alpha-and beta1-subunits. Circ Res. 1998;83:141–146. - PubMed
-
- Bennett ES, Smith BA, Harper JM. Voltage-gated Na+ channels confer invasive properties on human prostate cancer cells. Pflugers Arch. 2004;447:908–914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

