Altered peptide ligands can modify the Th2 T cell response to the immunodominant 161-175 peptide of LACK (Leishmania homolog for the receptor of activated C kinase)
- PMID: 19042022
- PMCID: PMC2659654
- DOI: 10.1016/j.molimm.2008.10.024
Altered peptide ligands can modify the Th2 T cell response to the immunodominant 161-175 peptide of LACK (Leishmania homolog for the receptor of activated C kinase)
Abstract
Following Leishmania major infection, the early LACK (Leishmania homolog of receptors for activated C kinase)-induced IL-4 response appears to determine disease susceptibility in BALB/c mice. Therefore, we sought to manipulate the pathogenic T cell responses to the immunodominant epitope with the use of altered peptide ligands (APLs). Conservative and non-conservative substitutions for each amino acid of the LACK 161-175 peptide determinant were tested for their stimulatory capacity in four different LACK-reactive T cell systems. From these results, we propose a likely LACK 163-171/I-A(d) core peptide register and show that APLs with changes at putative T cell receptor (TCR) contacts provide the greatest potential for immune deviation. In particular, the TCR-contact H164V APL expanded Th1 cells upon in vitro recall of naïve splenocytes from LACK-specific BV4 T cell receptor transgenic mice and stimulated IFN-gamma secretion from a Th2-committed LACK-reactive T cell line. We also observed that non-conservative substitutions flanking the core determinant had strong agonistic effects for proliferation and Th1/Th2 modulation. However, upon immunization, the H164V APL considerably downregulated proliferation and cytokine responses to the wild type LACK 161-175 peptide, while immunization with the weak agonist, MHC contact APL S171K, increased the IFN-gamma/IL-4 ratio to the wild type peptide. In these instances, a hyporesponsive T cell response to the wild-type peptide was achieved by immunizing with an APL possessing non-conservative substitutions at TCR contact sites, while immune deviation was accomplished using a weak-agonist APL that retained the core determinant. Thus, certain LACK-APLs are able to induce T cell responses with a protective phenotype in an infectious disease such as leishmaniasis.
Figures
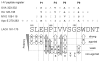

References
-
- Arnold PY, La Gruta NL, Miller T, Vignali KM, Adams PS, Woodland DL, Vignali DA. The majority of immunogenic epitopes generate CD4+ T cells that are dependent on MHC class II-bound peptide-flanking residues. J Immunol. 2002;169:739–49. - PubMed
-
- Carson RT, Vignali KM, Woodland DL, Vignali DA. T cell receptor recognition of MHC class II-bound peptide flanking residues enhances immunogenicity and results in altered TCR V region usage. Immunity. 1997;7:387–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

