Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects
- PMID: 19042039
- PMCID: PMC2637534
- DOI: 10.1016/j.tips.2008.10.003
Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects
Abstract
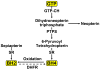
Tetrahydrobiopterin (BH4) is an essential cofactor required for the activity of endothelial nitric oxide (NO) synthase. Suboptimal concentrations of BH4 in the endothelium reduce the biosynthesis of NO, thus contributing to the pathogenesis of vascular endothelial dysfunction. Supplementation with exogenous BH4 or therapeutic approaches that increase endogenous amounts of BH4 can reduce or reverse endothelial dysfunction by restoring production of NO. Improvements in formulations of BH4 for oral delivery have stimulated clinical trials that test the efficacy of BH4 in the treatment of systemic hypertension, peripheral arterial disease, coronary artery disease, pulmonary arterial hypertension, and sickle cell disease. This review discusses ongoing progress in the translation of knowledge, accumulated in preclinical studies, into the clinical application of BH4 in the treatment of vascular diseases. This review also addresses the emerging roles of BH4 in the regulation of endothelial function and their therapeutic implications.
Figures

References
-
- Tayeh MA, Marletta MA. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate: tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989;264:19654–19658. - PubMed
-
- Kwon NS, et al. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989;264:20496–20501. - PubMed
-
- Mayer B, et al. Purification of a Ca2+/calmodulin-dependent nitric oxide synthase from porcine cerebellum. Cofactor-role of tetrahydrobiopterin. FEBS Lett. 1990;2771:215–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

