Molecular evolution, functional variation, and proposed nomenclature of the gene family that includes sphingomyelinase D in sicariid spider venoms
- PMID: 19042943
- PMCID: PMC2767091
- DOI: 10.1093/molbev/msn274
Molecular evolution, functional variation, and proposed nomenclature of the gene family that includes sphingomyelinase D in sicariid spider venoms
Abstract
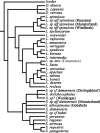
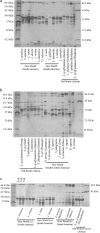
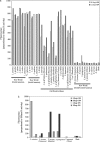
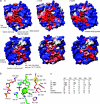
The venom enzyme sphingomyelinase D (SMase D) in the spider family Sicariidae (brown or fiddleback spiders [Loxosceles] and six-eyed sand spiders [Sicarius]) causes dermonecrosis in mammals. SMase D is in a gene family with multiple venom-expressed members that vary in functional specificity. We analyze molecular evolution of this family and variation in SMase D activity among crude venoms using a data set that represents the phylogenetic breadth of Loxosceles and Sicarius. We isolated a total of 190 nonredundant nucleotide sequences encoding 168 nonredundant amino acid sequences of SMase D homologs from 21 species. Bayesian phylogenies support two major clades that we name alpha and beta, within which we define seven and three subclades, respectively. Sequences in the alpha clade are exclusively from New World Loxosceles and Loxosceles rufescens and include published genes for which expression products have SMase D and dermonecrotic activity. The beta clade includes paralogs from New World Loxosceles that have no, or reduced, SMase D and no dermonecrotic activity and also paralogs from Sicarius and African Loxosceles of unknown activity. Gene duplications are frequent, consistent with a birth-and-death model, and there is evidence of purifying selection with episodic positive directional selection. Despite having venom-expressed SMase D homologs, venoms from New World Sicarius have reduced, or no, detectable SMase D activity, and Loxosceles in the Southern African spinulosa group have low SMase D activity. Sequence conservation mapping shows >98% conservation of proposed catalytic residues of the active site and around a plug motif at the opposite end of the TIM barrel, but alpha and beta clades differ in conservation of key residues surrounding the apparent substrate binding pocket. Based on these combined results, we propose an inclusive nomenclature for the gene family, renaming it SicTox, and discuss emerging patterns of functional diversification.
Figures

References
-
- Adams ME. Agatoxins: ion channel specific toxins from the American funnel web spider, Agelenopsis aperta. Toxicon. 2004;43:509–525. - PubMed
-
- Alegre AB, Meneses GO, Aguilar FPG. Peligrosidad de diez arenas communes en la costa central Peruana. Rev Per Ent. 1977;20:63–66.
-
- Appel MH, da Silveira RB, Chaim OM (11 co-authors) Identification and functional characterization of a novel dermonecrotic toxin (phospholipase D) from brown spider (Loxosceles intermedia) venom. Biochim Biophys Acta. 2008;1780:167–178. - PubMed
-
- Araujo SC, Castanheira P, Alvarenga LM, Mangili OC, Kalapothakis E, Chávez-Olórtegui C. Protection against dermonecrotic and lethal activities of Loxosceles intermedia spider venom by immunization with a fused recombinant protein. Toxicon. 2003;41:261–267. - PubMed
-
- Barbaro KC, Knysak I, Martins R, Hogan C, Winkel K. Enzymatic characterization, antigenic cross-reactivity and neutralization of dermonecrotic activity of five Loxosceles spider venoms of medical importance in the Americas. Toxicon. 2005;45:489–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

