A large number of protein expression changes occur early in life and precede phenotype onset in a mouse model for huntington disease
- PMID: 19043139
- PMCID: PMC2667354
- DOI: 10.1074/mcp.M800277-MCP200
A large number of protein expression changes occur early in life and precede phenotype onset in a mouse model for huntington disease
Abstract
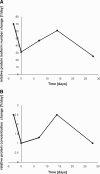
Huntington disease (HD) is fatal in humans within 15-20 years of symptomatic disease. Although late stage HD has been studied extensively, protein expression changes that occur at the early stages of disease and during disease progression have not been reported. In this study, we used a large two-dimensional gel/mass spectrometry-based proteomics approach to investigate HD-induced protein expression alterations and their kinetics at very early stages and during the course of disease. The murine HD model R6/2 was investigated at 2, 4, 6, 8, and 12 weeks of age, corresponding to absence of disease and early, intermediate, and late stage HD. Unexpectedly the most HD stage-specific protein changes (71-100%) as well as a drastic alteration (almost 6% of the proteome) in protein expression occurred already as early as 2 weeks of age. Early changes included mainly the up-regulation of proteins involved in glycolysis/gluconeogenesis and the down-regulation of the actin cytoskeleton. This suggests a period of highly variable protein expression that precedes the onset of HD phenotypes. Although an up-regulation of glycolysis/gluconeogenesis-related protein alterations remained dominant during HD progression, late stage alterations at 12 weeks showed an up-regulation of proteins involved in proteasomal function. The early changes in HD coincide with a peak in protein alteration during normal mouse development at 2 weeks of age that may be responsible for these massive changes. Protein and mRNA data sets showed a large overlap on the level of affected pathways but not single proteins/mRNAs. Our observations suggest that HD is characterized by a highly dynamic disease pathology not represented by linear protein concentration alterations over the course of disease.
Figures

Similar articles
-
Novel proteomic changes in brain mitochondria provide insights into mitochondrial dysfunction in mouse models of Huntington's disease.Mitochondrion. 2019 Jul;47:318-329. doi: 10.1016/j.mito.2019.03.004. Epub 2019 Mar 20. Mitochondrion. 2019. PMID: 30902619 Free PMC article.
-
Early decrease of type 1 cannabinoid receptor binding and phosphodiesterase 10A activity in vivo in R6/2 Huntington mice.Neurobiol Aging. 2014 Dec;35(12):2858-2869. doi: 10.1016/j.neurobiolaging.2014.06.010. Epub 2014 Jun 16. Neurobiol Aging. 2014. PMID: 25018107
-
Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington's disease mice.Hippocampus. 2010 May;20(5):621-36. doi: 10.1002/hipo.20658. Hippocampus. 2010. PMID: 19499586
-
Immediate-early gene response to methamphetamine, haloperidol, and quinolinic acid is not impaired in Huntington's disease transgenic mice.J Neurosci Res. 2002 Feb 1;67(3):372-8. doi: 10.1002/jnr.10100. J Neurosci Res. 2002. PMID: 11813242
-
Sphingolipids and impaired hypoxic stress responses in Huntington disease.Prog Lipid Res. 2023 Apr;90:101224. doi: 10.1016/j.plipres.2023.101224. Epub 2023 Mar 8. Prog Lipid Res. 2023. PMID: 36898481 Review.
Cited by
-
Novel proteomic changes in brain mitochondria provide insights into mitochondrial dysfunction in mouse models of Huntington's disease.Mitochondrion. 2019 Jul;47:318-329. doi: 10.1016/j.mito.2019.03.004. Epub 2019 Mar 20. Mitochondrion. 2019. PMID: 30902619 Free PMC article.
-
Brain Region and Cell Compartment Dependent Regulation of Electron Transport System Components in Huntington's Disease Model Mice.Brain Sci. 2021 Sep 24;11(10):1267. doi: 10.3390/brainsci11101267. Brain Sci. 2021. PMID: 34679332 Free PMC article.
-
Establishment of a mouse model with misregulated chromosome condensation due to defective Mcph1 function.PLoS One. 2010 Feb 16;5(2):e9242. doi: 10.1371/journal.pone.0009242. PLoS One. 2010. PMID: 20169082 Free PMC article.
-
Changes in the striatal proteome of YAC128Q mice exhibit gene-environment interactions between mutant huntingtin and manganese.J Proteome Res. 2012 Feb 3;11(2):1118-32. doi: 10.1021/pr200839d. Epub 2012 Jan 20. J Proteome Res. 2012. PMID: 22191580 Free PMC article.
-
The Role of Epigenetic Mechanisms in the Regulation of Gene Expression in the Nervous System.J Neurosci. 2016 Nov 9;36(45):11427-11434. doi: 10.1523/JNEUROSCI.2492-16.2016. J Neurosci. 2016. PMID: 27911745 Free PMC article. Review.
References
-
- Wexler, N. S., Rose, E. A., and Housman, D. E. ( 1991) Molecular approaches to hereditary diseases of the nervous system: Huntington's disease as a paradigm. Annu. Rev. Neurosci. 14, 503–529 - PubMed
-
- The Huntington's Disease Collaborative Research Group ( 1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 - PubMed
-
- McMurray, S. E., and McMurray, C. T. ( 2001) Huntington's disease. A sports star and a cook. Lancet 358, (suppl.) S38. - PubMed
-
- Rubinsztein, D. C., Leggo, J., Coles, R., Almqvist, E., Biancalana, V., Cassiman, J. J., Chotai, K., Connarty, M., Crauford, D., Curtis, A., Curtis, D., Davidson, M. J., Differ, A. M., Dode, C., Dodge, A., Frontali, M., Ranen, N. G., Stine, O. C., Sherr, M., Abbott, M. H., Franz, M. L., Graham, C. A., Harper, P. S., Hedreen, J. C., Jackson, A., Kaplan, J.-C., Losekoot, M., MacMillan, J. C., Morrison, P., Trottier, Y., Novelletto, A., Simpson, S. A., Theilmann, J., Whittaker, J. L., Folstein, S. E., Ross, C. A., and Hayden, M. R. ( 1996) Phenotypic characterization of individuals with 30–40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36–39 repeats. Am. J. Hum. Genet. 59, 16–22 - PMC - PubMed
-
- Bates, G. ( 2003) Huntingtin aggregation and toxicity in Huntington's disease. Lancet 361, 1642–1644 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

