A PI3-kinase-mediated negative feedback regulates neuronal excitability
- PMID: 19043547
- PMCID: PMC2581892
- DOI: 10.1371/journal.pgen.1000277
A PI3-kinase-mediated negative feedback regulates neuronal excitability
Abstract
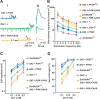
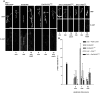
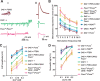
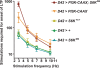
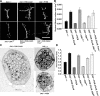
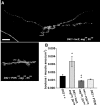
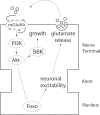
Use-dependent downregulation of neuronal activity (negative feedback) can act as a homeostatic mechanism to maintain neuronal activity at a particular specified value. Disruption of this negative feedback might lead to neurological pathologies, such as epilepsy, but the precise mechanisms by which this feedback can occur remain incompletely understood. At one glutamatergic synapse, the Drosophila neuromuscular junction, a mutation in the group II metabotropic glutamate receptor gene (DmGluRA) increased motor neuron excitability by disrupting an autocrine, glutamate-mediated negative feedback. We show that DmGluRA mutations increase neuronal excitability by preventing PI3 kinase (PI3K) activation and consequently hyperactivating the transcription factor Foxo. Furthermore, glutamate application increases levels of phospho-Akt, a product of PI3K signaling, within motor nerve terminals in a DmGluRA-dependent manner. Finally, we show that PI3K increases both axon diameter and synapse number via the Tor/S6 kinase pathway, but not Foxo. In humans, PI3K and group II mGluRs are implicated in epilepsy, neurofibromatosis, autism, schizophrenia, and other neurological disorders; however, neither the link between group II mGluRs and PI3K, nor the role of PI3K-dependent regulation of Foxo in the control of neuronal excitability, had been previously reported. Our work suggests that some of the deficits in these neurological disorders might result from disruption of glutamate-mediated homeostasis of neuronal excitability.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Davis GW. Homeostatic Control of Neural Activity: From Phenomenology to Molecular Design. Annu Rev Neurosci 2006 - PubMed
-
- Marder E, Prinz AA. Current compensation in neuronal homeostasis. Neuron. 2003;37:2–4. - PubMed
-
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

