Migratory dermal dendritic cells act as rapid sensors of protozoan parasites
- PMID: 19043558
- PMCID: PMC2583051
- DOI: 10.1371/journal.ppat.1000222
Migratory dermal dendritic cells act as rapid sensors of protozoan parasites
Abstract
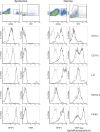
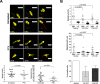
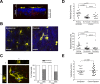
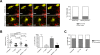
Dendritic cells (DC), including those of the skin, act as sentinels for intruding microorganisms. In the epidermis, DC (termed Langerhans cells, LC) are sessile and screen their microenvironment through occasional movements of their dendrites. The spatio-temporal orchestration of antigen encounter by dermal DC (DDC) is not known. Since these cells are thought to be instrumental in the initiation of immune responses during infection, we investigated their behavior directly within their natural microenvironment using intravital two-photon microscopy. Surprisingly, we found that, under homeostatic conditions, DDC were highly motile, continuously crawling through the interstitial space in a Galpha(i) protein-coupled receptor-dependent manner. However, within minutes after intradermal delivery of the protozoan parasite Leishmania major, DDC became immobile and incorporated multiple parasites into cytosolic vacuoles. Parasite uptake occurred through the extension of long, highly dynamic pseudopods capable of tracking and engulfing parasites. This was then followed by rapid dendrite retraction towards the cell body. DDC were proficient at discriminating between parasites and inert particles, and parasite uptake was independent of the presence of neutrophils. Together, our study has visualized the dynamics and microenvironmental context of parasite encounter by an innate immune cell subset during the initiation of the immune response. Our results uncover a unique migratory tissue surveillance program of DDC that ensures the rapid detection of pathogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Randolph GJ. Dendritic cell migration to lymph nodes: cytokines, chemokines, and lipid mediators. Semin Immunol. 2001;13:267–274. - PubMed
-
- Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. - PubMed
-
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. - PubMed
-
- Misslitz AC, Bonhagen K, Harbecke D, Lippuner C, Kamradt T, et al. Two waves of antigen-containing dendritic cells in vivo in experimental Leishmania major infection. Eur J Immunol. 2004;34:715–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

