PPARgamma and Agonists against Cancer: Rational Design of Complementation Treatments
- PMID: 19043603
- PMCID: PMC2586323
- DOI: 10.1155/2008/945275
PPARgamma and Agonists against Cancer: Rational Design of Complementation Treatments
Abstract
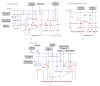
PPARgamma is a member of the ligand-activated nuclear receptor superfamily: its ligands act as insulin sensitizers and some are approved for the treatment of metabolic disorders in humans. PPARgamma has pleiotropic effects on survival and proliferation of multiple cell types, including cancer cells, and is now subject of intensive preclinical cancer research. Studies of the recent decade highlighted PPARgamma role as a potential modulator of angiogenesis in vitro and in vivo. These observations provide an additional facet to the PPARgamma image as potential anticancer drug. Currently PPARgamma is regarded as an important target for the therapies against angiogenesis-dependent pathological states including cancer and vascular complications of diabetes. Some of the studies, however, identify pro-angiogenic and tumor-promoting effects of PPARgamma and its ligands pointing out the need for further studies. Below, we summarize current knowledge of PPARgamma regulatory mechanisms and molecular targets, and discuss ways to maximize the beneficial activity of the PPARgamma agonists.
Figures


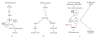
References
-
- Wang T, Xu J, Yu X, Yang R, Han ZC. Peroxisome proliferator-activated receptor γ in malignant diseases. Critical Reviews in Oncology/Hematology. 2006;58(1):1–14. - PubMed
-
- Bruemmer D, Blaschke F, Law RE. New targets for PPARγ in the vessel wall: implications for restenosis. International Journal of Obesity. 2005;29(supplement 1):S26–S30. - PubMed
-
- Margeli A, Kouraklis G, Theocharis S. Peroxisome proliferator activated receptor-γ (PPAR-γ) ligands and angiogenesis. Angiogenesis. 2003;6(3):165–169. - PubMed
-
- Panigrahy D, Shen LQ, Kieran MW, Kaipainen A. Therapeutic potential of thiazolidinediones as anticancer agents. Expert Opinion on Investigational Drugs. 2003;12(12):1925–1937. - PubMed
-
- Panigrahy D, Huang S, Kieran MW, Kaipainen A. PPARγ as a therapeutic target for tumor angiogenesis and metastasis. Cancer Biology and Therapy. 2005;4(7):687–693. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

