The mechanisms used by enteropathogenic Escherichia coli to control filopodia dynamics
- PMID: 19046338
- PMCID: PMC2688667
- DOI: 10.1111/j.1462-5822.2008.01254.x
The mechanisms used by enteropathogenic Escherichia coli to control filopodia dynamics
Abstract
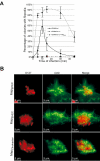
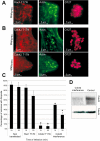
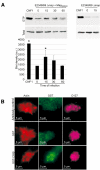
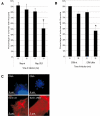
Enteropathogenic Escherichia coli (EPEC) subverts actin dynamics in eukaryotic cells by injecting effector proteins via a type III secretion system. First, WxxxE effector Map triggers transient formation of filopodia. Then, following recovery from the filopodial signals, EPEC triggers robust actin polymerization via a signalling complex comprising Tir and the adaptor proteins Nck. In this paper we show that Map triggers filopodia formation by activating Cdc42; expression of dominant-negative Cdc42 or knock-down of Cdc42 by siRNA impaired filopodia formation. In addition, Map binds PDZ1 of NHERF1. We show that Map-NHERF1 interaction is needed for filopodia stabilization in a process involving ezrin and the RhoA/ROCK cascade; expression of dominant-negative ezrin and RhoA or siRNA knock-down of RhoA lead to rapid elimination of filopodia. Moreover, we show that formation of the Tir-Nck signalling complex leads to filopodia withdrawal. Recovery from the filopodial signals requires phosphorylation of a Tir tyrosine (Y474) residue and actin polymerization pathway as both infection of cells with EPEC expressing TirY474S or infection of Nck knockout cells with wild-type EPEC resulted in persistence of filopodia. These results show that EPEC effectors modulate actin dynamics by temporal subverting the Rho GTPases and other actin polymerization pathways for the benefit of the adherent pathogen.
Figures
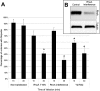
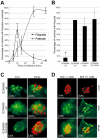
References
-
- Alto NM, Shao F, Lazar CS, Brost RL, Chua G, Mattoo S, et al. Identification of a bacterial type III effector family with G protein mimicry functions. Cell. 2006;124:133–145. - PubMed
-
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

