Engineering inclusion bodies for non denaturing extraction of functional proteins
- PMID: 19046444
- PMCID: PMC2630956
- DOI: 10.1186/1475-2859-7-34
Engineering inclusion bodies for non denaturing extraction of functional proteins
Abstract
Background: For a long time IBs were considered to be inactive deposits of accumulated target proteins. In our previous studies, we discovered IBs containing a high percentage of correctly folded protein that can be extracted under non-denaturing conditions in biologically active form without applying any renaturation steps. In order to widen the concept of correctly folded protein inside IBs, G-CSF (granulocyte colony stimulating factor) and three additional proteins were chosen for this study: GFP (Green fluorescent protein), His7dN6TNF-alpha (Truncated form of Tumor necrosis factor alpha with an N-terminal histidine tag) and dN19 LT-alpha (Truncated form of Lymphotoxin alpha).
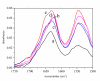
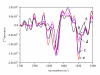
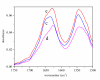
Results: Four structurally different proteins that accumulate in the bacterial cell in the form of IBs were studied, revealing that distribution of each target protein between the soluble fraction (cytoplasm) and insoluble fraction (IBs) depends on the nature of the target protein.Irrespective of the folding pattern of each protein, spectroscopy studies have shown that proteins in IBs exhibit similar structural characteristics to the biologically active pure protein when produced at low temperature. In the case of the three studied proteins, G-CSF, His7DeltaN6TNF-alpha, and GFP, a significant amount of protein could be extracted from IBs with 0.2% N-lauroyl sarcosine (NLS) and the proteins retained biological activity although no renaturation procedure was applied.
Conclusion: This study shows that the presence of biologically active proteins inside IBs is more general than usually believed. A large amount of properly folded protein is trapped inside IBs prepared at lower temperatures. This protein can be released from IBs with mild detergents under non-denaturing conditions. Therefore, the active protein can be obtained from such IBs without any renaturation procedure. This is of great importance for the biopharmaceutical industry. Furthermore, such IBs composed of active proteins could also be used as pure nanoparticles in diagnostics, as biocatalysts in enzymatic processes, or even as biopharmaceuticals.
Figures





References
-
- Rinas U, Tsai LB, Lyons D, Fox GM, Stearns G, Fieschko J, et al. Cysteine to serine substitutions in basic fibroblast growth factor: effect on inclusion body formation and proteolytic susceptibility during in vitro refolding. Biotechnology (N Y) 1992;10:435–440. doi: 10.1038/nbt0492-435. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

