Role of Src signal transduction pathways in scatter factor-mediated cellular protection
- PMID: 19047046
- PMCID: PMC2658051
- DOI: 10.1074/jbc.M807497200
Role of Src signal transduction pathways in scatter factor-mediated cellular protection
Abstract
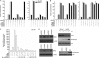
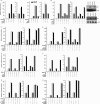
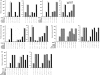
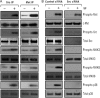
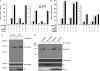
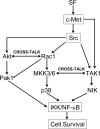
Scatter factor (SF) (hepatocyte growth factor) is a pleiotrophic cytokine that accumulates in tumors, where it may induce invasion, angiogenesis, and chemoresistance. We have studied the mechanisms by which SF and its receptor (c-Met) protect cells against the DNA-damaging agent adriamycin (ADR) as a model for chemoresistance of SF/c-Met-overexpressing tumors. Previous studies identified a phosphatidylinositol 3-kinase/c-Akt/Pak1/NF-kappaB cell survival pathway in DU-145 prostate cancer and Madin-Darby canine kidney epithelial cells. Here we studied Src signaling pathways involved in SF cell protection. Src enhanced basal and SF stimulated NF-kappaB activity and SF protection against ADR, in a manner dependent upon its kinase and Src homology 3 domains; and endogenous Src was required for SF stimulation of NF-kappaB activity and cell protection. The ability of Src to enhance SF stimulation of NF-kappaB activity was due, in part, to its ability to stimulate Akt and IkappaB kinase activity; and Src-mediated stimulation of NF-kappaB was due, in part, to a Rac1/MKK3/6/p38 pathway and was Akt-dependent. SF caused the activation of Src and the Rac1 effector Pak1. Furthermore, SF induced activating phosphorylations of MKK3, MKK6, and p38 within the c-Met signalsome in an Src-dependent manner. The NF-kappaB-inducing kinase was found to act downstream of TAK1 (transforming growth factor-beta-activated kinase 1) as a mediator of SF- and Src-stimulated NF-kappaB activity. Finally, the Src/Rac1/MKK3/6/p38 and Src/TAK1/NF-kappaB-inducing kinase pathways exhibited cross-talk at the level of MKK3. These findings delineate some novel signaling pathways for SF-mediated resistance to ADR.
Figures



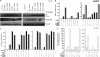
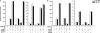
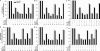
Similar articles
-
Ras effector pathways modulate scatter factor-stimulated NF-kappaB signaling and protection against DNA damage.Oncogene. 2007 Jul 19;26(33):4774-96. doi: 10.1038/sj.onc.1210271. Epub 2007 Feb 12. Oncogene. 2007. PMID: 17297451
-
Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection.Oncogene. 2005 Mar 3;24(10):1749-66. doi: 10.1038/sj.onc.1208327. Oncogene. 2005. PMID: 15688034
-
The multisubstrate adapter Gab1 regulates hepatocyte growth factor (scatter factor)-c-Met signaling for cell survival and DNA repair.Mol Cell Biol. 2001 Aug;21(15):4968-84. doi: 10.1128/MCB.21.15.4968-4984.2001. Mol Cell Biol. 2001. PMID: 11438654 Free PMC article.
-
Epithelial growth factor receptor-activated nuclear factor κB signaling and its role in epithelial growth factor receptor-associated tumors.Cancer J. 2013 Nov-Dec;19(6):461-7. doi: 10.1097/PPO.0000000000000001. Cancer J. 2013. PMID: 24270344 Review.
-
Anti-cancer therapeutic strategies based on HGF/MET, EpCAM, and tumor-stromal cross talk.Cancer Cell Int. 2022 Aug 19;22(1):259. doi: 10.1186/s12935-022-02658-z. Cancer Cell Int. 2022. PMID: 35986321 Free PMC article. Review.
Cited by
-
Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions.Cell Mol Life Sci. 2012 Nov;69(22):3765-805. doi: 10.1007/s00018-012-1019-0. Epub 2012 Jun 29. Cell Mol Life Sci. 2012. PMID: 22744749 Free PMC article. Review.
-
Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer.J Natl Cancer Inst. 2012 Sep 5;104(17):1306-19. doi: 10.1093/jnci/djs319. Epub 2012 Aug 21. J Natl Cancer Inst. 2012. PMID: 22911670 Free PMC article.
-
Targeting tyrosine kinases and autophagy in prostate cancer.Horm Cancer. 2011 Feb;2(1):38-46. doi: 10.1007/s12672-010-0053-3. Epub 2010 Dec 2. Horm Cancer. 2011. PMID: 21350583 Free PMC article. Review.
-
Mouse hepatocyte overexpression of NF-κB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis.Hepatology. 2014 Dec;60(6):2065-76. doi: 10.1002/hep.27348. Epub 2014 Oct 29. Hepatology. 2014. PMID: 25088600 Free PMC article.
-
Potential therapeutics specific to c-MET/RON receptor tyrosine kinases for molecular targeting in cancer therapy.Acta Pharmacol Sin. 2010 Sep;31(9):1181-8. doi: 10.1038/aps.2010.106. Epub 2010 Aug 9. Acta Pharmacol Sin. 2010. PMID: 20694025 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

