Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors
- PMID: 19047122
- PMCID: PMC2652879
- DOI: 10.1158/1078-0432.CCR-08-1223
Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors
Abstract
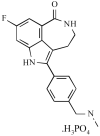
Purpose: One mechanism of tumor resistance to cytotoxic therapy is repair of damaged DNA. Poly(ADP-ribose) polymerase (PARP)-1 is a nuclear enzyme involved in base excision repair, one of the five major repair pathways. PARP inhibitors are emerging as a new class of agents that can potentiate chemotherapy and radiotherapy. The article reports safety, efficacy, pharmacokinetic, and pharmacodynamic results of the first-in-class trial of a PARP inhibitor, AG014699, combined with temozolomide in adults with advanced malignancy.
Experimental design: Initially, patients with solid tumors received escalating doses of AG014699 with 100 mg/m2/d temozolomide x 5 every 28 days to establish the PARP inhibitory dose (PID). Subsequently, AG014699 dose was fixed at PID and temozolomide escalated to maximum tolerated dose or 200 mg/m2 in metastatic melanoma patients whose tumors were biopsied. AG014699 and temozolomide pharmacokinetics, PARP activity, DNA strand single-strand breaks, response, and toxicity were evaluated.
Results: Thirty-three patients were enrolled. PARP inhibition was seen at all doses; PID was 12 mg/m2 based on 74% to 97% inhibition of peripheral blood lymphocyte PARP activity. Recommended doses were 12 mg/m2 AG014699 and 200 mg/m2 temozolomide. Mean tumor PARP inhibition at 5 h was 92% (range, 46-97%). No toxicity attributable to AG014699 alone was observed. AG014699 showed linear pharmacokinetics with no interaction with temozolomide. All patients treated at PID showed increases in DNA single-strand breaks and encouraging evidence of activity was seen.
Conclusions: The combination of AG014699 and temozolomide is well tolerated, pharmacodynamic assessments showing proof of principle of the mode of action of this new class of agents.
Figures

References
-
- Hoeijmakers JH. Genone maintenance mechanisms for preventing cancer. Nature. 2001;411:360–74. - PubMed
-
- Heinen CD, Schmutte C, Fishel R. DNA repair and tumorigenesis: lessons from hereditary cancer syndromes. Cancer Biol Ther. 2002;1:477–85. - PubMed
-
- Risinger MA, Groden J. Crosslinks and crosstalk: human cancer syndromes and DNA repair defects. Cancer Cell. 2004;6:539–45. - PubMed
-
- Gatti L, Zunino F. Overview of tumor cell chemoresistance mechanisms. Methods Mol Med. 2005;111:127–48. - PubMed
-
- Madhusudan S, Middleton MR. The emerging role of DNA repair proteins as predictive, prognostic and therapeutic targets in cancer. Cancer Treat Rev. 2005;31:603–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

