The hypocretins as sensors for metabolism and arousal
- PMID: 19047201
- PMCID: PMC2670020
- DOI: 10.1113/jphysiol.2008.164400
The hypocretins as sensors for metabolism and arousal
Abstract
Sleep disturbances are associated with hormonal imbalances and may result in metabolic disorders including obesity and diabetes. Therefore, circuits controlling both sleep and metabolism are likely to play a role in these physiopathological conditions. The hypocretin (Hcrt) system is a strong candidate for mediating both sleep and metabolic imbalances because Hcrt neurons are sensitive to metabolic hormones, including leptin and ghrelin, and modulate arousal and goal-orientated behaviours. This review discusses the role of Hcrt neurons as a sensors of energy balance and arousal and proposes new ways of probing local hypothalamic circuits regulating sleep and metabolism with unprecedented cellular specificity and temporal resolution.
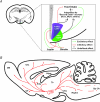
Figures


References
-
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. - PMC - PubMed
-
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–S156. - PubMed
-
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

