The human CDK8 subcomplex is a histone kinase that requires Med12 for activity and can function independently of mediator
- PMID: 19047373
- PMCID: PMC2630685
- DOI: 10.1128/MCB.00993-08
The human CDK8 subcomplex is a histone kinase that requires Med12 for activity and can function independently of mediator
Abstract
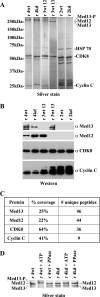
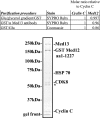
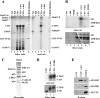
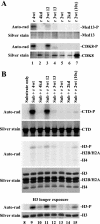
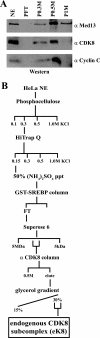
The four proteins CDK8, cyclin C, Med12, and Med13 can associate with Mediator and are presumed to form a stable "CDK8 subcomplex" in cells. We describe here the isolation and enzymatic activity of the 600-kDa CDK8 subcomplex purified directly from human cells and also via recombinant expression in insect cells. Biochemical analysis of the recombinant CDK8 subcomplex identifies predicted (TFIIH and RNA polymerase II C-terminal domain [Pol II CTD]) and novel (histone H3, Med13, and CDK8 itself) substrates for the CDK8 kinase. Notably, these novel substrates appear to be metazoan-specific. Such diverse targets imply strict regulation of CDK8 kinase activity. Along these lines, we observe that Mediator itself enables CDK8 kinase activity on chromatin, and we identify Med12--but not Med13--to be essential for activating the CDK8 kinase. Moreover, mass spectrometry analysis of the endogenous CDK8 subcomplex reveals several associated factors, including GCN1L1 and the TRiC chaperonin, that may help control its biological function. In support of this, electron microscopy analysis suggests TRiC sequesters the CDK8 subcomplex and kinase assays reveal the endogenous CDK8 subcomplex--unlike the recombinant submodule--is unable to phosphorylate the Pol II CTD.
Figures
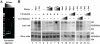


References
-
- Akoulitchev, S., S. Chuikov, and D. Reinberg. 2000. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 407102-106. - PubMed
-
- Andrau, J., L. van de Pasch, P. Lijnzaad, T. Bijma, M. G. Koerkamp, J. van de Peppel, M. Werner, and F. C. P. Holstege. 2006. Genome-wide location of the coactivator Mediator: binding without activation and transient cdk8 interaction on DNA. Mol. Cell 22179-192. - PubMed
-
- Anest, V., J. L. Hanson, P. C. Cogswell, K. A. Steinbrecher, B. D. Strahl, and A. S. Baldwin. 2003. A nucleosomal function for IκB kinase-α in NF-κB-dependent gene expression. Nature 423659-663. - PubMed
-
- Behrends, C., C. A. Langer, R. Boteva, U. M. Bottcher, M. J. Stemp, G. Schaffar, B. V. Rao, A. Giese, H. Kretzschmar, K. Siegers, and F. U. Hartl. 2006. Chaperonin TRiC promotes the assembly of polyQ expansion proteins into nontoxic oligomers. Mol. Cell 23887-897. - PubMed
-
- Borggrefe, T., R. Davis, H. Erdjument-Bromage, P. Tempst, and R. D. Kornberg. 2002. A complex of the Srb8, -9, -10, and -11 transcriptional regulatory proteins from yeast. J. Biol. Chem. 27744202-44207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
