Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses
- PMID: 19047440
- PMCID: PMC2605227
- DOI: 10.1084/jem.20082292
Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses
Abstract
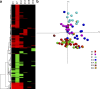
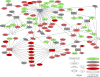
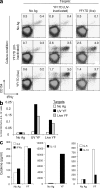
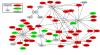
Correlates of immune-mediated protection to most viral and cancer vaccines are still unknown. This impedes the development of novel vaccines to incurable diseases such as HIV and cancer. In this study, we have used functional genomics and polychromatic flow cytometry to define the signature of the immune response to the yellow fever (YF) vaccine 17D (YF17D) in a cohort of 40 volunteers followed for up to 1 yr after vaccination. We show that immunization with YF17D leads to an integrated immune response that includes several effector arms of innate immunity, including complement, the inflammasome, and interferons, as well as adaptive immunity as shown by an early T cell response followed by a brisk and variable B cell response. Development of these responses is preceded, as demonstrated in three independent vaccination trials and in a novel in vitro system of primary immune responses (modular immune in vitro construct [MIMIC] system), by the coordinated up-regulation of transcripts for specific transcription factors, including STAT1, IRF7, and ETS2, which are upstream of the different effector arms of the immune response. These results clearly show that the immune response to a strong vaccine is preceded by coordinated induction of master transcription factors that lead to the development of a broad, polyfunctional, and persistent immune response that integrates all effector cells of the immune system.
Figures





References
-
- Plotkin, S., and W. Orenstein. 2004. Vaccines. WB Saunders, Philadelphia.
-
- McMichael, A.J. 2006. HIV vaccines. Annu. Rev. Immunol. 24:227–255. - PubMed
-
- Monath, T.P. 2004. Yellow fever vaccine. In Vaccines. S. Plotkin and W. Orenstein, editors. WB Saunders, Philadelphia. 1095–1176.
-
- Bauer, J.H. 1931. The duration of passive immunity in yellow fever. Am. J. Trop. Med. Hyg. 11:451.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

