Specific interactions between the viral coreceptor CXCR4 and the biguanide-based compound NB325 mediate inhibition of human immunodeficiency virus type 1 infection
- PMID: 19047650
- PMCID: PMC2630669
- DOI: 10.1128/AAC.00866-08
Specific interactions between the viral coreceptor CXCR4 and the biguanide-based compound NB325 mediate inhibition of human immunodeficiency virus type 1 infection
Abstract
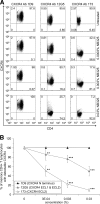
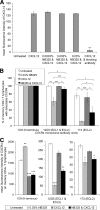
The present studies were conducted to better define the mechanism of action of polyethylene hexamethylene biguanide (PEHMB) (designated herein as NB325), which was shown in previous studies to inhibit infection by the human immunodeficiency virus type 1 (HIV-1). Fluorescence-activated flow cytometric analyses of activated human CD4(+) T lymphocytes exposed to NB325 demonstrated concentration-dependent reductions in CXCR4 epitope recognition in the absence of altered recognition of selected CD4 or CD3 epitopes. NB325 also inhibited chemotaxis of CD4(+) T lymphocytes induced by the CXCR4 ligand CXCL12. However, NB325 did not cause CXCR4 internalization (unlike CXCL12) and did not interfere with CXCL12 binding. Additional flow cytometric analyses using antibodies with distinct specificities for extracellular domains of CXCR4 demonstrated that NB325 specifically interfered with antibody binding to extracellular loop 2 (ECL2). This interaction was confirmed using competitive binding analyses, in which a peptide derived from CXCR4 ECL2 competitively inhibited NB325-mediated reductions in CXCR4 epitope recognition. Collectively, these results demonstrate that the biguanide-based compound NB325 inhibits HIV-1 infection by specifically interacting with the HIV-1 coreceptor CXCR4.
Figures



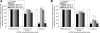
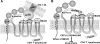
Similar articles
-
Cervicovaginal safety of the formulated, biguanide-based human immunodeficiency virus type 1 (HIV-1) inhibitor NB325 in a murine model.J Biomed Biotechnol. 2011;2011:941061. doi: 10.1155/2011/941061. Epub 2011 Oct 24. J Biomed Biotechnol. 2011. PMID: 22131821 Free PMC article.
-
Persistent interactions between biguanide-based compound NB325 and CXCR4 result in prolonged inhibition of human immunodeficiency virus type 1 infection.Antimicrob Agents Chemother. 2010 May;54(5):1965-72. doi: 10.1128/AAC.00934-09. Epub 2010 Mar 15. Antimicrob Agents Chemother. 2010. PMID: 20231400 Free PMC article.
-
Structure-activity relationships of polybiguanides with activity against human immunodeficiency virus type 1.Biomed Pharmacother. 2010 Dec;64(10):723-32. doi: 10.1016/j.biopha.2010.10.001. Epub 2010 Nov 4. Biomed Pharmacother. 2010. PMID: 21106331 Free PMC article.
-
The CXCL12/CXCR4 axis as a therapeutic target in cancer and HIV-1 infection.Curr Med Chem. 2011;18(4):497-512. doi: 10.2174/092986711794480159. Curr Med Chem. 2011. PMID: 21143114 Review.
-
Maraviroc, a CCR5 coreceptor antagonist that blocks entry of human immunodeficiency virus type 1.Pharmacotherapy. 2009 Mar;29(3):295-304. doi: 10.1592/phco.29.3.295. Pharmacotherapy. 2009. PMID: 19249948 Review.
Cited by
-
Microscopic Examination of Polymeric Monoguanidine, Hydrochloride-Induced Cell Membrane Damage in Multidrug-Resistant Pseudomonas aeruginosa.Polymers (Basel). 2017 Aug 31;9(9):398. doi: 10.3390/polym9090398. Polymers (Basel). 2017. PMID: 30965707 Free PMC article.
-
Cervicovaginal safety of the formulated, biguanide-based human immunodeficiency virus type 1 (HIV-1) inhibitor NB325 in a murine model.J Biomed Biotechnol. 2011;2011:941061. doi: 10.1155/2011/941061. Epub 2011 Oct 24. J Biomed Biotechnol. 2011. PMID: 22131821 Free PMC article.
-
Pharmacotherapy of pediatric and adolescent HIV infection.Ther Clin Risk Manag. 2009 Jun;5(3):469-84. doi: 10.2147/tcrm.s4594. Epub 2009 Jun 22. Ther Clin Risk Manag. 2009. PMID: 19707256 Free PMC article.
-
Small molecule inhibitors of CXCR4.Theranostics. 2013;3(1):47-75. doi: 10.7150/thno.5376. Epub 2013 Jan 15. Theranostics. 2013. PMID: 23382786 Free PMC article. Review.
-
Gene Editing of HIV-1 Co-receptors to Prevent and/or Cure Virus Infection.Front Microbiol. 2018 Dec 17;9:2940. doi: 10.3389/fmicb.2018.02940. eCollection 2018. Front Microbiol. 2018. PMID: 30619107 Free PMC article. Review.
References
-
- Amara, A., O. Lorthioir, A. Valenzuela, A. Magerus, M. Thelen, M. Montes, J. L. Virelizier, M. Delepierre, F. Baleux, H. Lortat-Jacob, and F. Arenzana-Seisdedos. 1999. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J. Biol. Chem. 274:23916-23925. - PubMed
-
- Balzarini, J., and L. Van Damme. 2007. Microbicide drug candidates to prevent HIV infection. Lancet 369:787-797. - PubMed
-
- Bleul, C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, J. Sodroski, and T. A. Springer. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

