Fatty acid metabolism: target for metabolic syndrome
- PMID: 19047759
- PMCID: PMC2674721
- DOI: 10.1194/jlr.R800079-JLR200
Fatty acid metabolism: target for metabolic syndrome
Abstract
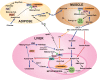
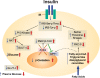
Fatty acids are a major energy source and important constituents of membrane lipids, and they serve as cellular signaling molecules that play an important role in the etiology of the metabolic syndrome. Acetyl-CoA carboxylases 1 and 2 (ACC1 and ACC2) catalyze the synthesis of malonyl-CoA, the substrate for fatty acid synthesis and the regulator of fatty acid oxidation. They are highly regulated and play important roles in the energy metabolism of fatty acids in animals, including humans. They are presently considered as an attractive target to regulate the human diseases of obesity, diabetes, cancer, and cardiovascular complications. In this review we discuss the role of fatty acid metabolism and its key players, ACC1 and ACC2, in animal evolution and physiology, as related to health and disease.
Figures
References
-
- Wakil S. J. 1989. The fatty acid synthase: a proficient multifunctional enzyme. Biochemistry. 28 4523–4530. - PubMed
-
- Wakil S. J., E. R. Titchener, and D. M. Gibson. 1958. Evidence for the participation of biotin in the enzymic synthesis of fatty acids. Biochim. Biophys. Acta. 29 225–226. - PubMed
-
- Abu-Elheiga L., D. B. Almarza-Ortega, A. Baldini, and S. J. Wakil. 1997. Human acetyl-CoA carboxylase 2: molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. J. Biol. Chem. 272 10669–10677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

