Immunogenicity of fractional doses of tetravalent a/c/y/w135 meningococcal polysaccharide vaccine: results from a randomized non-inferiority controlled trial in Uganda
- PMID: 19048025
- PMCID: PMC2584372
- DOI: 10.1371/journal.pntd.0000342
Immunogenicity of fractional doses of tetravalent a/c/y/w135 meningococcal polysaccharide vaccine: results from a randomized non-inferiority controlled trial in Uganda
Abstract
Background: Neisseria meningitidis serogroup A is the main causative pathogen of meningitis epidemics in sub-Saharan Africa. In recent years, serogroup W135 has also been the cause of epidemics. Mass vaccination campaigns with polysaccharide vaccines are key elements in controlling these epidemics. Facing global vaccine shortage, we explored the use of fractional doses of a licensed A/C/Y/W135 polysaccharide meningococcal vaccine.
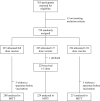
Methods and findings: We conducted a randomized, non-inferiority trial in 750 healthy volunteers 2-19 years old in Mbarara, Uganda, to compare the immune response of the full dose of the vaccine versus fractional doses (1/5 or 1/10). Safety and tolerability data were collected for all subjects during the 4 weeks following the injection. Pre- and post-vaccination sera were analyzed by measuring serum bactericidal activity (SBA) with baby rabbit complement. A responder was defined as a subject with a > or =4-fold increase in SBA against a target strain from each serogroup and SBA titer > or =128. For serogroup W135, 94% and 97% of the vaccinees in the 1/5- and 1/10-dose arms, respectively, were responders, versus 94% in the full-dose arm; for serogroup A, 92% and 88% were responders, respectively, versus 95%. Non-inferiority was demonstrated between the full dose and both fractional doses in SBA seroresponse against serogroups W135 and Y, in total population analysis. Non-inferiority was shown between the full and 1/5 doses for serogroup A in the population non-immune prior to vaccination. Non-inferiority was not shown for any of the fractionate doses for serogroup C. Safety and tolerability data were favourable, as observed in other studies.
Conclusions: While the advent of conjugate A vaccine is anticipated to largely contribute to control serogroup A outbreaks in Africa, the scale-up of its production will not cover the entire "Meningitis Belt" target population for at least the next 3 to 5 years. In view of the current shortage of meningococcal vaccines for Africa, the use of 1/5 fractional doses should be considered as an alternative in mass vaccination campaigns.
Trial registration: ClinicalTrials.gov NCT00271479.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Lapeyssonnie L. [Comparative epidemiologic study of meningococcic cerebrospinal meningitis in temperate regions and in the meningitis belt in Africa. Attempt at synthesis]. Med Trop. 1968;28:709–720. - PubMed
-
- World Health Organization. Detecting meningococcal meningitis epidemics in highly-endemic African countries. Wkly Epidemiol Rec. 2000;75:306–309. - PubMed
-
- Ouedraogo-Traore R, Hoiby EA, Sanou I, Sangare L, Kyelem N, et al. Molecular characteristics of Neisseria meningitidis strains isolated in Burkina Faso in 2001. Scand J Infect Dis. 2002;34:804–807. - PubMed
-
- World Health Organization. Meningococcal disease, serogroup W135, Burkina Faso. Wkly Epidemiol Rec. 2002;77:152–155. - PubMed
-
- Zombre S, Hacen MM, Ouango G, Sanou S, Adamou Y, et al. The outbreak of meningitis due to Neisseria meningitidis W135 in 2003 in Burkina Faso and the national response: main lessons learnt. Vaccine. 2007;25(Suppl 1):A69–A71. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

