Variant HNF1 modulates epithelial plasticity of normal and transformed ovary cells
- PMID: 19048126
- PMCID: PMC2586698
- DOI: 10.1593/neo.81004
Variant HNF1 modulates epithelial plasticity of normal and transformed ovary cells
Abstract
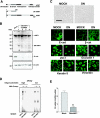
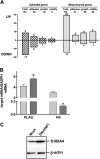
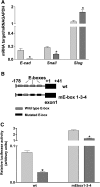
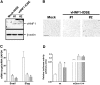
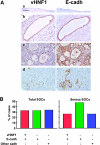
Ovarian carcinoma arises from the ovarian surface epithelium, which undergoes phenotypic changes characteristic of müllerian epithelium during the first stages of tumorigenesis. The variant isoform of the hepatocyte nuclear factor 1 (vHNF1) is a transcription factor involved in the development of tissues derived from the müllerian duct. Here, we show that vHNF1 knockdown in two ovarian carcinoma cell lines, SKOV3 and IGROV1, leads to reduced E-cadherin (E-cadh) expression and decreased proliferation rate. Accordingly, SKOV3 cells ectopically expressing a dominant-negative (DN) vHNF1 mutant undergo an epithelial-mesenchymal-like transition, acquiring a spindle-like morphology, loss of E-cadh, and disrupted cell-cell contacts. Gene expression profiling of DNvHNF1 cells on the basis of a newly compiled list of epithelial-mesenchymal transition-related genes revealed a correlation between vHNF1 loss-of-function and acquisition of the mesenchymal phenotype. Indeed, phenotypic changes were associated with increased Slug transcription and functionality. Accordingly, vHNF1-transfected immortalized ovarian surface epithelial cells showed down-regulation of Snail and Slug transcripts. In DNvHNF1-transfected SKOV3 cells, growth rate decreased, and in vHNF1-transfected immortalized ovarian surface epithelial cells, growth rate increased. By immunohistochemistry, we found a strong association of vHNF1 with E-cadh in clear cell and in a subset of serous carcinomas, data that could potentially contribute in distinguishing different types of ovarian tumors. Our results may help in understanding the biology of ovarian carcinoma, identifying early detection markers, and opening potential avenues for therapeutic intervention.
Figures

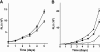
References
-
- Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(Suppl 2):S19–S32. - PubMed
-
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–288. - PubMed
-
- Strumane K, Van Roy F, Berx G. The role of E-cadherin in epithelial differentiation and cancer progression. Recent Res Devel Cell Biochem. 2003;1:33–37.
-
- Richmond PJM, Karayiannakis AJ, Nagafuchi A, Kaisary AV, Pignatelli M. Aberrant E-cadherin and α-catenin expression in prostate cancer: correlation with patient survival. Cancer Res. 1997;57:3189–3193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
