At what costs will screening with CT colonography be competitive? A cost-effectiveness approach
- PMID: 19048626
- PMCID: PMC2859672
- DOI: 10.1002/ijc.24025
At what costs will screening with CT colonography be competitive? A cost-effectiveness approach
Abstract
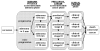
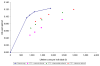
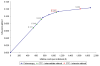
The costs of computed tomographic colonography (CTC) are not yet established for screening use. In our study, we estimated the threshold costs for which CTC screening would be a cost-effective alternative to colonoscopy for colorectal cancer (CRC) screening in the general population. We used the MISCAN-colon microsimulation model to estimate the costs and life-years gained of screening persons aged 50-80 years for 4 screening strategies: (i) optical colonoscopy; and CTC with referral to optical colonoscopy of (ii) any suspected polyp; (iii) a suspected polyp >or=6 mm and (iv) a suspected polyp >or=10 mm. For each of the 4 strategies, screen intervals of 5, 10, 15 and 20 years were considered. Subsequently, for each CTC strategy and interval, the threshold costs of CTC were calculated. We performed a sensitivity analysis to assess the effect of uncertain model parameters on the threshold costs. With equal costs ($662), optical colonoscopy dominated CTC screening. For CTC to gain similar life-years as colonoscopy screening every 10 years, it should be offered every 5 years with referral of polyps >or=6 mm. For this strategy to be as cost-effective as colonoscopy screening, the costs must not exceed $285 or 43% of colonoscopy costs (range in sensitivity analysis: 39-47%). With 25% higher adherence than colonoscopy, CTC threshold costs could be 71% of colonoscopy costs. Our estimate of 43% is considerably lower than previous estimates in literature, because previous studies only compared CTC screening to 10-yearly colonoscopy, where we compared to different intervals of colonoscopy screening.
Figures


Comment in
-
The diminutive lesion versus the advanced adenoma: Which is the real target of CT colonography screening?Int J Cancer. 2009 Sep 1;125(5):1238; author reply 1239-40. doi: 10.1002/ijc.24478. Int J Cancer. 2009. PMID: 19479994 No abstract available.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126:1674–80. - PubMed
-
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. - PubMed
-
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. - PubMed
-
- Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–37. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

