Leuko-polymersomes
- PMID: 19048993
- PMCID: PMC2714229
- DOI: 10.1039/b717821b
Leuko-polymersomes
Abstract
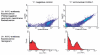
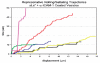
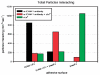
Polymersomes are vesicles whose membranes are comprised of self-assembled amphiphilic block co-polymers. Synthetic control of block co-polymer chemistry provides an advantageous diversity of polymersome functions, ranging from tunable materials strength, superior encaspulation of hydrophobic and hydrophilic drugs and optical dyes, and facile functionalization. We have exploited polymersome tunability to make leuko-polymersomes: polymersomes with the adhesive properties of leukocytes. By functionalizing the terminal groups on the outer shell of the vesicle with biotin, we have used modular avidin-biotin chemistry to attach adhesion ligands that mimic the two critical adhesion pathways that leukocytes utilize to achieve adhesion in the fast fluid flow of blood vessels--selectins and integrins. We demonstrate that adhesion is specific and is supported at hydrodynamic flow rates at which leukocytes adhere. We envision the use of such particles for monitoring or treating inflammation, cancer and cardiovascular disease.
Figures



References
-
- Hillmyer MA, Bates FS. Macromolecules. 1996;29:6994–7002.
-
- Longo ML, Waring AJ, Gordon LM, Hammer DA. Langmuir. 1998;14:2385–2395.
-
- Angelova MI, Soléau S, Méléard P, Faucon JF, Bothorel P. Prog. Colloid Polym. Sci. 1992;89:127–131.
-
- Hajduk DA, Kossuth MB, Hillmyer MA, Bates FS. J. Phys. Chem. B. 1998;102:4269–4276.
-
- Won Y-Y, Davis HT, Bates FS. Science. 1999;283:960–963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

