Copper catalyzed enantioselective intramolecular aminooxygenation of alkenes
- PMID: 19049311
- PMCID: PMC2674573
- DOI: 10.1021/ja806585m
Copper catalyzed enantioselective intramolecular aminooxygenation of alkenes
Abstract
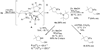
The copper-catalyzed enantioselective intramolecular aminooxygenation of alkenes is reported herein. This is the first report of an enantioselective intramolecular alkene aminooxygenation process. N-Arylsulfonyl-2-allylanilines and 4-pentenylarylsulfonamides cyclize in high yield and with good enantioselectivity, providing new chiral methyleneoxy-functionalized dihydroindolines and pyrrolidines. Tetramethylaminopyridyl radical (TEMPO) serves as both the source of the oxygen and the stoichiometric oxidant. These reactions are catalyzed by copper(II) triflate, complexed with (4S,5R)-Bis-Phbox. The unprotected aminoalcohols can be obtained by sequential dissolving metal reductions of the N-S and O-N bonds.
Figures
References
-
- Bergmeier SC. Tetrahedron. 2000;26:2561.
-
-
Reviews of aminohydroxylation processes: O’Brien P. Angew. Chem. Int. Ed. Engl. 1999;38:326. Bolm C, Hildebrand JP, Muniz K. In: Catalytic Asymmetric Synthesis. 2nd ed. Ojima I, editor. Wiley-VCH; 2000. pp. 412–424. Schlingloff G, Sharpless BK. In: Asymmetric Oxidation Reactions. Katsuki T, editor. Oxford University Press; 2001. pp. 104–114. Nilov D, Reiser O. Adv. Synth. Catal. 2002;344:1169. Bodkin JK, McLeod MD. J. Chem. Soc. Perkin Trans. 1. 2002:2733.
-
-
- Donohoe TJ, Churchill GH, Wheelhouse KMP, Glossop PA. Angew. Chem. Int. Ed. Engl. 2006;45:8025. - PubMed
- Donohoe TJ, Chughtai MJ, Klauber DJ, Griffin D, Campbell AD. J. Am. Chem. Soc. 2006;128:2514. - PubMed
- Donohoe TJ, Bataille CJR, Gattrell W, Kloeges J, Rossignol E. Org. Lett. 2007;9:1725. - PubMed
-
-
For other intramolecular aminooxygenation reactions, see: Noack M, Gottlich R. Chem. Commun. 2002:536. Chikkanna D, Han H. Synlett. 2004:2311. Correa A, Tellitu I, Dominguez E, SanMartin R. J. Org. Chem. 2006;71:8316. Cochran BM, Michael FE. Org. Lett. 2008;10:5093. Mahoney JM, Smith CR, Johnston JN. J. Am. Chem. Soc. 2005;127:1354. (f) for recent copper and palladium-catalyzed intermolecular aminooxygenation reactions, see supporting information.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous