Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue
- PMID: 19049324
- PMCID: PMC2659511
- DOI: 10.1359/jbmr.081210
Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue
Abstract
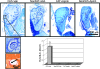
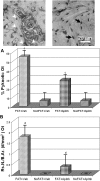
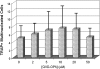
Osteocyte apoptosis is spatially and temporally linked to bone fatigue-induced microdamage and to subsequent intracortical remodeling. Specifically, osteocytes surrounding fatigue microcracks in bone undergo apoptosis, and those regions containing apoptotic osteocytes co-localize exactly with areas subsequently resorbed by osteoclasts. Here we tested the hypothesis that osteocyte apoptosis is a key controlling step in the activation and/or targeting of osteoclastic resorption after bone fatigue. We carried out in vivo fatigue loading of ulna from 4- to 5-mo-old Sprague-Dawley rats treated with an apoptosis inhibitor (the pan-caspase inhibitor Q-VD-OPh) or with vehicle. Intracortical bone remodeling and osteocyte apoptosis were quantitatively assessed by standard histomorphometric techniques on day 14 after fatigue. Continuous exposure to Q-VD-OPh completely blocked both fatigue-induced apoptosis and the activation of osteoclastic resorption, whereas short-term caspase inhibition during only the first 2 days after fatigue resulted in >50% reductions in both osteocyte apoptosis and bone resorption. These results (1) show that osteocyte apoptosis is necessary to initiate intracortical bone remodeling in response to fatigue microdamage, (2) indicate a possible dose-response relationship between the two processes, and (3) suggest that early apoptotic events after fatigue-induced microdamage may play a substantial role in determining the subsequent course of tissue remodeling.
Figures

References
-
- Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech. 1985;18:189–200. - PubMed
-
- Enlow DH. Functions of the Haversian system. Am J Anat. 1962;110:269–305. - PubMed
-
- Parfitt AM. Bone age, mineral density, and fatigue damage. Calcif Tissue Int. 1993;53(Suppl 1):S82–S85. - PubMed
-
- Schaffler MB. Role of bone turnover in microdamage. Osteoporos Int. 2003;14(Suppl 5):73–80. - PubMed
-
- Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23:275–281. - PubMed

