Alpha-Synuclein conformation affects its tyrosine-dependent oxidative aggregation
- PMID: 19049426
- PMCID: PMC2645542
- DOI: 10.1021/bi801884z
Alpha-Synuclein conformation affects its tyrosine-dependent oxidative aggregation
Abstract
Oxidative stress and aggregation of the protein alpha-synuclein are thought to be key factors in Parkinson's disease. Previous work shows that cytochrome c with H(2)O(2) causes tyrosine-dependent in vitro peroxidative aggregation of proteins, including alpha-synuclein. Here, we examine the role of each of alpha-synuclein's four tyrosine residues and how the protein's conformation affects covalent oxidative aggregation. When alpha-synuclein adopts a collapsed conformation, tyrosine 39 is essential for wild-type-like covalent aggregation. This lone N-terminal tyrosine, however, is not required for wild-type-like covalent aggregation in the presence of a denaturant or when alpha-synuclein is present in noncovalent fibrils. We also show that preformed oxidative aggregates are not incorporated into noncovalent fibrils. These data provide insight into how dityrosine may be formed in Lewy bodies seen in Parkinson's disease.
Figures



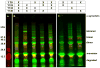

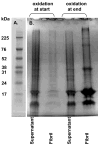
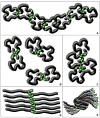
References
-
-
National Parkinson’s foundation.
-
-
- Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Hashimoto M, Takeda A, Hsu LJ, Takenouchi T, Masliah E. Role of cytochrome c as a stimulator of alpha-synuclein aggregation in Lewy body disease. J Biol Chem. 1999;274:28849–28852. - PubMed
-
- D’Andrea MR, Ilyin S, Plata-Salaman CR. Abnormal patterns of microtubule-associated protein-2 (map-2) immunolabeling in neuronal nuclei and Lewy bodies in Parkinson’s disease substantia nigra brain tissues. Neuroscience Letters. 2001;306:137–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

