Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors
- PMID: 19050032
- PMCID: PMC2724921
- DOI: 10.1093/brain/awn330
Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors
Abstract
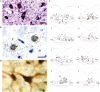
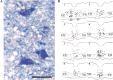
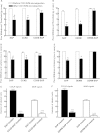
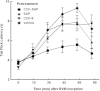
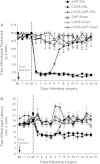
Descending input from the rostral ventromedial medulla (RVM) provides positive and negative modulation of spinal nociceptive transmission and has been proposed to be critical for maintaining neuropathic pain. This study tests the hypothesis that neuropathic pain requires the activity of a subset of RVM neurons that are distinguished by co-expression of mu opioid receptor (MOR) and cholecystokinin type 2 receptor (CCK2). Using male Sprague-Dawley rats, we demonstrate that discrete RVM neurons express MOR and CCK2; over 80% of these cells co-express both receptors. Agonist-directed cell lesion in the RVM with the cytotoxin, saporin, using either CCK-saporin to target CCK receptor expressing cells, or dermorphin-saporin to target MOR expressing cells, resulted in concomitant loss of CCK2 and MOR expressing cells, did not alter the basal sensory thresholds but abolished the hyperalgesia induced by microinjection of CCK into the RVM. The findings suggest that these CCK2-MOR co-expressing RVM neurons facilitate pain and can be directly activated by CCK input to the RVM. Furthermore, lesion of these RVM neurons did not affect the initial development of neuropathic pain in the hind paw upon injury to the sciatic nerve, but the abnormal pain states were short lived such that by about day 9 the sensory thresholds had reverted to pre-injury baselines despite the existing neuropathy. These data support our hypothesis and identify CCK2-MOR co-expressing neurons in the RVM as potential therapeutic targets for neuropathic pain.
Figures

References
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci meth. 1994;53:55–63. - PubMed
-
- Fields HL. Nociceptive transmission: the pain system. Science. 1985;228:1522. - PubMed
-
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

