Differential requirements for retinal degeneration slow intermolecular disulfide-linked oligomerization in rods versus cones
- PMID: 19050038
- PMCID: PMC2640204
- DOI: 10.1093/hmg/ddn406
Differential requirements for retinal degeneration slow intermolecular disulfide-linked oligomerization in rods versus cones
Abstract
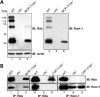
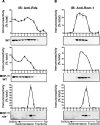
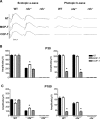
It is commonly assumed that the ultrastructural organization of the rim region of outer segment (OS) discs in rods and lamellae in cones requires functional retinal degeneration slow/rod outer segment membrane protein 1 (Rds/Rom-1) complexes. Cysteine-150 (C150) in Rds has been implicated in intermolecular disulfide bonding essential for functional Rds complexes. Transgenic mice containing the Rds C150S mutation (C150S-Rds) failed to form higher-order Rds oligomers, although interactions between C150S-Rds and Rom-1 occurred in rods, but not in cones. C150S-Rds mice exhibited marked early-onset reductions in cone function and abnormal OS structure. In contrast, C150S-Rds expression in rods partly rescued the rds(+/-) phenotype. Although C150S-Rds was detected in the OSs in rods and cones, a substantial percentage of C150S-Rds and cone opsins were mislocalized to different cellular compartments in cones. The results of this study provide novel insights into the importance of C150 in Rds oligomerization and the differences in Rds requirements in rods versus cones. The apparent OS structural differences between rods and cones may cause cones to be more susceptible to the elimination of higher-order Rds/Rom-1 oligomers (e.g. as mediated by mutation of the Rds C150 residue).
Figures




References
-
- Corless J.M., Fetter R.D., Costello M.J. Structural features of the terminal loop region of frog retinal rod outer segment disk membranes. I. Organization of lipid components. J. Comp. Neurol. 1987;257:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

